In mid May 2000, this video was taken of some of the varieties of zooplankton found by diving on an ebb tide at the end of the docks on the North side of Race Rocks.
Category Archives: Invertebrate
A dive with Dr. Scott Wallace for the Discovery Channel,
 Scott Wallace did research in 1997 and 1998 at Race Rocks with Pearson College divers. He studied the population dynamics of the Northern Abalone,Haliotis kamtchatkana
Scott Wallace did research in 1997 and 1998 at Race Rocks with Pearson College divers. He studied the population dynamics of the Northern Abalone,Haliotis kamtchatkana
His research was done as part of a PhD thesis in Resource Management from the University of British Columbia in Vancouver :
Wallace, S. S. 1999. Fisheries Impacts on Marine Ecosystems and Biological Diversity: The role for marine protected areas in British Columbia. Ph.D. Dissertation. The University of British Columbia. Pp. 198.
In May of 2000, he returned to Race Rocks for a dive with Garry and Hana and an interview with Stephanie Paine and Director Julia Nunes for the Discovery Channel. In this video he demonstrates the measurement technique he used in his research.
 Conservation Biology, Vol 13 No 4, August, 1999, pages 882-887.
Conservation Biology, Vol 13 No 4, August, 1999, pages 882-887.
ABSTRACT: :Marine reserves have been suggested as tools for assisting the management of fisheries by protecting vulnerable marine species from overexploitation. Although there is a theoretical basis for believing that marine reserves may serve as management tools, there are few marine reserves in the world in which to test their effectiveness. My research evaluated three forms of marine reserve on the south coast of Vancouver Island, British Columbia, Canada. I used northern abalone (Haliotis kamtschatkana), a severely depleted shellfish in this region, as an indicator of the effectiveness of the reserves. Abalone populations in eight sites receiving different degrees of spatial protection were counted and measured in situ during the spring of 1996 and 1997. In all sites with enforced harvest closures, populations of abalone were greater, and one site with nearly 40 years of protection had on average much larger (older) abalone. Reproductive output, as a function of abundance and size, was also greater in the enforced reserve areas. Larval dispersal from reserves, and hence the benefit to exploited areas, was not formally surveyed. Nevertheless, the results of my study, combined with knowledge of present abalone populations, life history, and regional hydrodynamics, suggest that establishment of reserves is justified in the absence of perfect knowledge of larval dispersal.
This video was taken by Garry Fletcher of Scott as he was involved in measuring abalone at Race Rocks:
Articles published by Scott Wallace:
Wallace, Scott, S. 1999, Evaluating the Effects of Three Forms of Marine Reserve on Northern Abalone Populations in British Columbia, Canada. Conservation Biology, Vol 13 No 4, August, 1999, pages 882-887.
Out of Sight, Out of Mind, and Almost out of Time:
Invertebrate Phyla at Race Rocks
“Invertebrate Phyla at Race Rocks” is an introductory piece on some of the invertebrate phyla that our divers encounter underwater . This was one of the first streaming videos done by the Pearson College divers in April 2000. It was taken by Rowena and Shamsher on a Sony Digital camera, and edited by Hannah and Garry on a G4 Macintosh Computer using iMovie.
The Ecological Niche of Anthopleura elegantissima at Race Rocks”
“The Ecological Niche of
Anthopleura elegantissima at Race Rocks”
by: Santiago Salinas
Candidate number: 0034 – 119
Subject: Biology Best Language Spanish
Student , Lester B. Pearson College of the Pacific
Submitted as partial fulfillment for the International Baccalaureate diploma program, January 2000
As with any other species at Race Rocks, Anthopleura elegantissima is an important member of the ecosystem to which it belongs. By knowing its ecological niche, trends can be analyzed, niche overlapping or other predictions may be made, particularly, for example, if new species are introduced.
?Since the statistical device suggested that the three populations are organized in the same way, a general description of the ecological niche was given. The species prefer a temperature range of 11-13ƒ C, the ideal elevation span goes from 1.5 to 2.8 meters, thus, it is an inter tidal species, and finally, the preferable time underwater was found to be 5 to 15 hours.
____________________________________________________________
?The Problem ………..?………….9 ?
?Purpose and Background of the Study………9
?Hypothesis …………………………….…..9
?Assumptions …………………………..…..1
?Limitations …………………………….….10
?Definition of Terms ………………………11
Review of Literature and Related Research
?Introduction, Information about the Organism …..13
?The Theory ………………………………….15
?Research Results in Related Areas ……16
Research Design and Procedures
?The Setting and Population of the Stud…18
?Field Work …………………19?
Instruments…………………………21
?Statistical Techniques Used ………22
Analysis of Data
?
Introduction …………………………………….23
?Findings ………………………………………23
Conclusions and Recommendations for Further Study
?
Interpretation and Implications of the Findings……………40
?Recommendations …………………………….42
Appendix
Bibliography
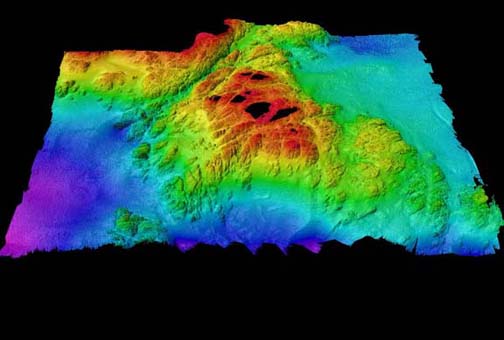
Figure 1. Topographic representation of Race Rocks…….12
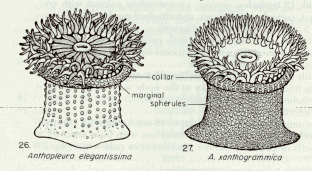
Figure 2. Representation of Anthopleura elegantissima and Anthopleura xanthogrammica …………………..14
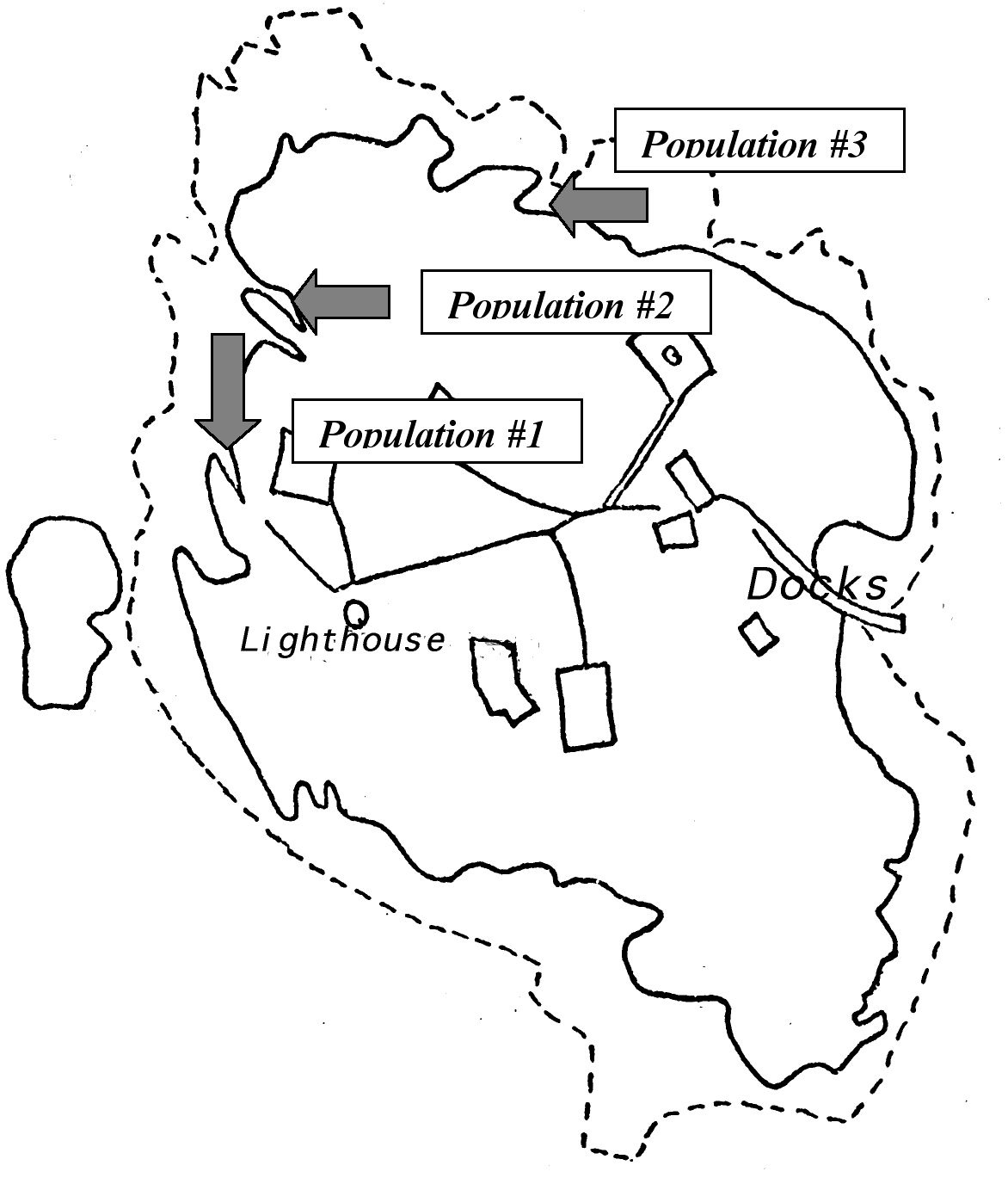
Figure 3. Location of Populations at Race Rocks…18
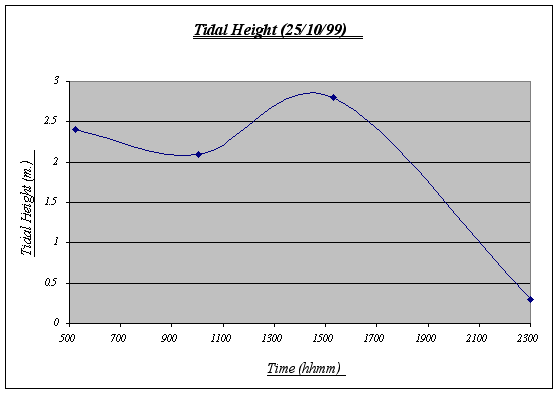
Figure 4. Tidal Height graph ………………………. …23
Figure 5. Terrain Gradient graph (Population 1) ……25
Figure 6. Abundance graph (Population 1) …………..26
Figure 7. Elevation against # of Organisms graph (Population 1.27
Figure 8. % of Organisms at different Temperatures graph (Population 1) ……..28
Figure 9. Terrain Gradient graph (Population 2) …30
Figure 10. Abundance graph (Population 2)………31
Figure 11. Elevation against # of Organisms graph (Population 2) 32
Figure 12. % of Organisms at different Temperatures graph (Population 2) ……33
Figure 13. Terrain Gradient graph (Population 3) …….35
Figure 14. Abundance graph (Population 3) ……………36
Figure 15. Elevation against # of Organisms graph (Population 3)…37
Figure 16. % of Organisms at different Temperatures graph (Population 3) ……………………38
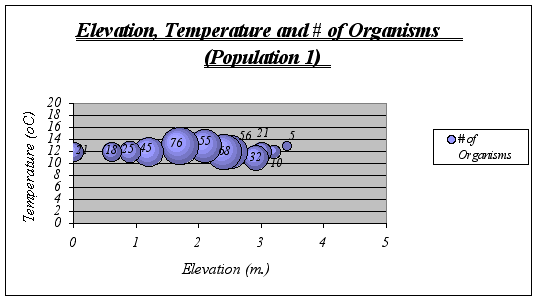
Figure 17. Elevation, Temperature and # of Organisms graph (Population 1) …………………40
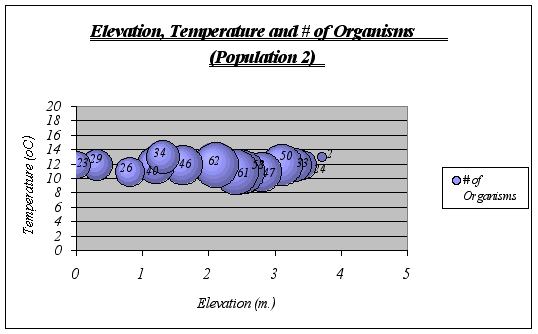
?Figure 18. Elevation, Temperature and # of Organisms graph (Population 2) …………………41
Figure 19. Elevation, Temperature and # of Organisms graph (Population 3) ………………….42
Tables
Table 1. Data Collection for Population 1 ………24
Table 2. Data Collection for Population 2 ………29
Table 3. Data Collection for Population 3…………34
?Finally, I would like to thank all the organisms at Race Rocks, especially Anthopleura elegantissima, for their patience and understanding of my investigations and for not complaining from my sometimes careless techniques.
Introduction
The Problem: Anthopleura elegantissima (common name: aggregating anemone) plays, along with all other biotic and abiotic components, an important role in the ecosystem to which it belongs. It is a highly valuable member of many food webs and participates in symbiotic relationships with other species. Taking this information into account, it would be useful to know facts about A. elegantissima in order to be able to predict and analyze trends in the ecosystem so as to gain an insightful knowledge about the species and its relation to the ecosystem found at Race Rocks.
Purpose and Background of the Study: To achieve the goals presented, a wide range of proposals were identified, leading to the decision to investigate the ecological niche as a focused and clear plan. The final design involved testing three populations at different locations of the island with the purpose of determining the preferred range of abiotic conditions for A. elegantissima. Four very significant variables were examined, all of them intimately related with intertidal zonation. These four variables were: the slope of the gradient, the tidal height, the time covered by sea water and the rock temperature. Carrying out the field work in three different sites allowed room for generalized conclusions about the species to be made.
Hypothesis: A null hypothesis for the Mann-Whitney test was formulated: “the three different locations are not inter-related and the similarities that may occur are merely coincidental.” If rejected, some conjectures about the species had to be formed. Given that Anthopleura elegantissima is a low inter tidal species, its range of positioning would not be between 1 to 3 meters. A hypothesis was made based on the fact that sea water is generally at about 10ƒ C, thus the rock temperature was not expected to be similar to this.
Assumptions: One of the major assumptions made was that the tide tables for Victoria, British Columbia, Canada are the same as these for Race Rocks. In theory this may not be true since Race Rocks is located approximately 10 km. away from Victoria. Therefore, there might exist a slight difference in tidal heights between the two zones.
Limitations: The entire experiment and data collection was done on the 27th of October, 1999, due to the limiting factor of relative low tides occurring in day-light hours. Hence the experiment could not be repeated on another day. Therefore, the research reveals the ecological niche of Anthopleura elegantissima at a fixed point in time and not the variations or changes in its distribution over a period of time. Furthermore, based on these results, the general ecological niche of the species cannot be concluded since all the data was gathered in a specific place, Race Rocks, which is a distinct site due to its location.
Another major limitation is that the “ecological niche” is an abstract term and therefore, the ecological niche of a species can never be fully represented. What was done in this case was to narrow the aspects to be considered and try to work with them by relating each factor to the others to acquire an approximation of the ecological niche. In this essay, four abiotic components were measured in order to obtain an insight to the ecological niche of Anthopleura elegantissima.
Definition of Terms: A number of terms should be defined at this point in order to ensure a clear understanding of this essay. One of these terms is population, which is defined as: all members of a species living in a particular area and making up one breeding group (Kucera, 1978). This is of particular importance since a similar species, Anthopleura xanthogrammica, may be found on the island, a phenomenon which would cause some distortions in the results if they are counted as Anthopleura elegantissima. Tides – the gravitational effects of the sun and moon on the oceans of the earth – are also a fundamental pivot in intertidal life. Tides along the Pacific coast of North America are of the mixed semidiurnal type; that is, there is a pronounced difference between the levels to which two successive low tides fall, and a lesser, but still apparent, difference between the levels reached by two successive high tides (Carefoot, 1977). Lastly, the most important term to be defined is ecological niche. The niche of a population or species is its functional role in an ecosystem. Using a human analogy, the niche is the species’ profession or way of life whereas the habitat is where this way of life is carried on — its address. The way a population responds to the various characteristics of its habitat is part of this population’s way of life and, therefore, of its niche. Hutchinson was the first to formally quantify the niche concept in terms of geometric space. The level of activity describes the ability of the individual to exploit the resources in a given level of each environmental factor (Odum, 1963). Then the niche space occupied by the species is the 3-dimensional space actually occupied by all individuals (Rickleffs, 1996). An empirical model (Box and Draper, 1989) can be obtained by the empirical determination of niche occupancy (number of individuals, in this case) in terms of n environmental variables (slope, tidal height, time covered and rock temperature).
Introduction, Information about the Organism: The field work was conducted at Race Rocks, Southern Vancouver Island, British Columbia, Canada. This area was chosen for ecological reserve status because of its unique richness and diversity of marine life. Race Rocks is ideally located to receive a constant supply of plankton swept past by almost continuous strong currents (up to 7 knots) . This provides nourishment for a complex group of underwater organisms.
One of the many organisms found at Race Rocks are sea anemones. Sea anemones belong to the phylum known as the Cnidaria, from the cnida or stinging cells that are present in this major group of animals that also include corals, jellyfish, hydroids, medusae, and sea fans. Sea anemones, corals and their allies form the class know as the Anthozoa. Anthopleura elegantissima (Phylum Cnidaria, Class Anthozoa, Subclass Zoantharia, Order Actiniatia, Family Actiniidae) is abundant on rock faces or boulders, in tide pools or crevices, on wharf pilings, singly or in dense aggregations (Smith and Carlton, 1975). It is a species characteristic of middle intertidal zone of semi protected rocky shores of both bays and outer coast from Alaska to Baja California. Aggregating individuals do not exceed 6 centimeters in column diameter and 8 centimeters across the tentacular crown. The column is light green to white, and twice as long as wide when extended, with longitudinal rows of adhesive tubercles (verrucae) often bearing attached debris (Carefoot, 1977). The species presents numerous short tentacles, in five or more cycles, which are variously colored. Anthopleura elegantissima reproduces both sexually and asexually. In sexual reproduction, ova are present as early as February and grow steadily until their release in July; the ovarian is then resorbed and new eggs do not appear until the following February. Sperm are released through the summer. The asexual reproduction occurs by longitudinal fission. This process results in aggregations or clones of anemones pressed together in concentrations of several hundred per square meter. Anthopleura elegantissima feeds on copepods, isopods, amphipods, and other small animals that contact the tentacles. On the other hand, it is preyed upon by the nudibranch Aeolidia papillosa, which usually attacks the column, by the snail Epitonium tinctum, which attacks the tips of the tentacles, and by sea stars such as Dermasterias imbricata that can engulf an entire small anemone. Moreover, in some anemones, small pink amphipods, Allogaussia recondita, make a home in the gastro vascular cavity (Carefoot, 1977).
The theory: In 1957, G. E. Hutchinson defined the niche concept formally. One could describe the activity range along every dimension of the environment. Physical and chemical factors such as temperature, humidity, salinity, and oxygen concentration, as well as biological factors such as prey species and resting background against which an individual may escape of predators, could be determined. Each of these dimensions could be thought of as one of the n-dimensions in space. Visualizing a space with more than three dimensions is difficult, thus the concept of the n-dimensional niche is an abstraction. We may, however, deal with multi-dimensional concepts mathematically and statistically, depicting their essence by physical or graphical representations in three or fewer dimensions. Moreover, Ricklefs notes that “… for example, a graph relating biological activity to a single environmental gradient represents the distribution of a species’ activity along one niche dimension. The level of activity, whether oxygen metabolism as a function of temperature or consumption rate as function of prey size, conveys the ability of an individual to exploit resources in a particular part of the niche space and, conversely, the degree to which the environment can support the population of that species. In two dimensions the individuals niche may be depicted as a hill with contours representing the various levels of biological activity. In three dimensions, we must think of a cloud in space whose density conveys niche utilization. Beyond three the mind boggles.”
To be more precise, it should be recognized that there are three different definitions for the term niche. The first one (also known as “niche as community function”) comes from Elton (1927) defining it as the animal’s place in the biotic environment, its relation to food and enemies. The second definition is called “niche in the species” and reveals that a specific set of capabilities for extracting resources, for surviving hazard, and for competing, coupled with a corresponding set of needs (Colinvaux, 1982). The most used and known is the one defined by Hutchinson, which was explained previously.
Research Results in Related Areas: Even though only one research paper was found containing information about Anthopleura elegantissima at Race Rocks, many investigations have been carried out with Anthopleura elegantissima and its physiology. The paper obtained on Anthopleura elegantissima at Race Rocks (Zahid, 1987) tries to detail the distribution of the species in one crack by two statistical methods (Plotless and Poisson techniques). It is concluded that A. elegantissima is an intertidal organism showing a clumped distribution. The clonal form, being lower mid-intertidal is exposed to sunlight and air much more than the solitary form in the sub-tidal zone. Hence, the clumped distribution is very useful and is also an important factor in reducing desiccation and water loss, as clumping reduces the surface area exposed to light.
The Setting and Population of the Study: The field work was designed to take place in three different locations of Race Rocks (see Figure 4), in order to gain a more detailed examination of the ecological niche of Anthopleura elegantissima in this island. Another reason for doing so was well explained by Odum: “It is also true that the same species may function differently —that is, occupy different niches- in different habitats or geographical regions.” The three places exhibit different environmental conditions and, therefore, the species may experience changes in its distribution. These changes could be current flow (which is in and of itself a major contributor to tidal life), light exposure, and even different rock composition.
A hazardous inconvenience that had to be overcome before doing the field work was to be able to distinguish among the two major types of sea anemones at Race Rocks, Anthopleura elegantissima and Anthopleura xanthogrammica. In order to achieve this, a key book was consulted. In the book, the distinctive characteristics between the two are described (see Appendix.)
Field Work: The complete field work took place on the 27th of October, 1999 due to the limiting factor of low tide level. The priorities were the elevation of the terrain and the number of organisms per quadrat in view of the fact that the tidal level was constantly altering, making the measurements inaccurate otherwise. The rock temperature was taken once these two sets of values had been gathered from the three locations. For the sake of help, a line transect was set in each crack to make the data collection uncomplicated and feasible.
Elevation: With the aim of measuring the elevation of the terrain for each population, a rudimentary, home-made apparatus was created. Due to the fact that the topography of the shore is extremely irregular at Race Rocks, it is not possible to assume that the elevation is a straight line. Thus, to have a detailed insight of Anthopleura elegantissima —or any other intertidal organism- at Race Rocks, an imperative factor to be considered is elevation. To approach this, the first action taken was to delimit the transect (generally a straight line along which observations are made in a systematic fashion) and the quadrats (starting from where the tidal level equals 0 meter). Since there was not a zero meter tide predicted, it was decided to start at 0945 approximately —tidal height equal to 2- and calculate the zero tide level. To accomplish the task, a two meter stick was used perpendicular to the sea water since tidal height is a vertical measure of water. Following this, a 4 meter stick was put where the tidal level equals 0 meter -quadrat number 15- (perpendicular to the sea water) and, with the help of a rope, a triangle was formed between the stick, the rope and the last quadrat —number 1. Once the triangle was finished, the elevation existing between quadrat number 1 and number 15 is known by the distance in the stick from the land to the conjuncture of it and the rope (for example, 3.9 in the first population). The subsequent measurements were much easier to carry since only a meter stick and a measuring tape were needed. Starting from quadrat number 1, a meter was measured with the measuring tape along the land. Then, the meter stick was put in such a manner that it formed a 90ƒ angle with the top of the quadrat, giving a number (0.3 in the first population). Therefore, 3.1 minus 0.3 equals 2.6, the elevation for quadrat number 2). So on and so forth the procedure was repeated until quadrat number 15 was reached.
Number of organisms: A very important feature involving the number of organisms per quadrat is the quadrat’s size. It was proven by Grey-Smith (1952) that the size of a quadrat could actually determine some erroneous conclusions in a population by using a series of progressively larger quadrats to measure the distribution in an artificial situation in which individuals were represented by colored disks. A reasonably good size for the quadrats was estimated to be 0.5 by 0.5 meters, given personal observation. Once the quadrats were sorted out, the counting took place. Only if more than 75 % of the organism was inside the quadrat was it counted. A difficult aspect of the counting was to differentiate individuals from the same group clone or aggregation. This difficulty was expected since the clones are held together very tightly and because at this time of the year young anemones are developing their bodies (as fertilization occurs during summer).
Rock temperature: This process was relatively simple compared to the previous two. It consisted of using the thermometer in small crevices in rocks —for each quadrat- in order to get the rock temperature.
?Time underwater: Aided by the tide table for the day (starting at 0500 and finishing at 2300), it is possible to calculate how much time a certain elevation is exposed to sea water. Assume that it is desirable to know the underwater time of a quadrat at 2.5. Then, we trace a line at 2.5 and the area under the curve will indicate the time that quadrat was covered by water.
Instruments Used: A simple technological apparatus was used for the field work. A measuring tape, a meter stick, a four meter stick, the tide tables for Victoria, BC, a thermometer, and a rope were all the required instruments.
Statistical Techniques Used: The technique used to verify that the distribution of the species was not random or by chance was the Mann-Whitney test. This non-parametric tool (meaning there are no specific distributional assumptions required) is sometimes called Wilcoxon test or rank sum test. This test relies on a special kind of transformation that replaces each observation by its rank in the combined sample. The purpose of this is to transform the data to a scale that eliminates the importance of the population distribution altogether (Ramsey and Schafer, 1997). In order to make it easier and more accurate, a web-page (VassarStats) was utilized to perform the calculations and the statistics values.
Introduction: The data will be presented by population and not by factor. This is aimed to help the understanding of the ecological niche of Anthopleura elegantissima at Race Rocks in a detailed and comprehensible fashion. Three separate populations were examined on different parts of the island The populations are numbered (1, 2 and 3) referring to a certain strip (see Figure 4.)
Findings: The Mann-Whitney test was used to determine whether or not there is a general trend between the three populations. Two populations were tested at a time, therefore, three runs of the test were conducted using the number of organisms as the variable to be ranked. Using VassarStats, an U value was calculated: 140.5 for populations 1 and 2, 96.5 for populations 1 and 3, and 44 for populations 2 and 3. These U values were then checked in the significance levels table:
By this, it is possible to conclude that, although it is impossible to be absolutely certain that the different is not due to chance, the probability is sufficiently small for it to be considered negligible. Thus, the null hypothesis can be rejected and assume that there are similarities among the three populations. Bearing that in mind, the variables should now be tested to determine the ecological niche of Anthopleura elegantissima at Race Rocks.
?Tidal Height:
The variations of the tidal height of the day are normal and reflect the constant water movement that take place in the ocean affecting inter tidal life. Based on this chart, the underwater time will be calculated. Note that the chart begins at 0500 and ends at 2300 (therefore, total time underwater = 18:00).
Population # 1:
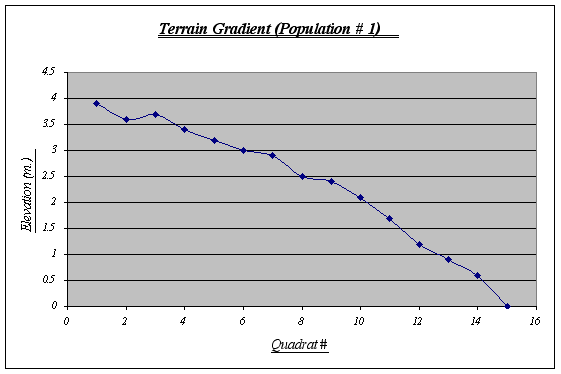
The terrain gradient for population number 1 is the typical slope and is also the most regular among the three. It has a steep drop at the end (quadrat # 15) where it meets the sea water at 0 m. (tidal level). This gradient features some small tide pools and is sometimes covered by kelp beds. By personal observations, I could say that it is the strip with the highest level of species diversity on it.
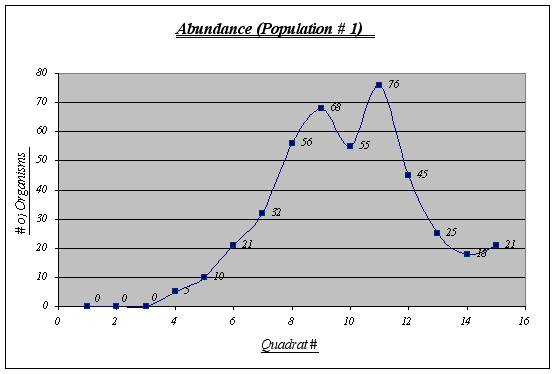
Even though this is not the most abundant population (432 individuals), it features the largest number of individuals per quadrat (76) and has an extremely large number of Anthopleura elegantissima at a certain level of the gradient. It should also be noted that no individuals were found in the first three quadrats and there were not many before quadrat 6. The number of organisms was measured in quadrats of 0.25 square meters.
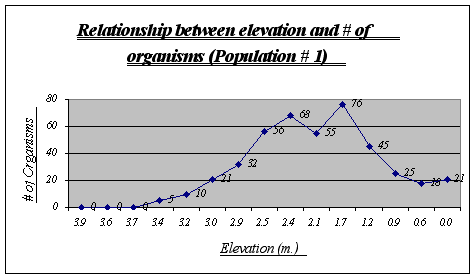
This graph clearly shows the relationship between elevation and number of organisms. It is easy to recognize that the preferred place for Anthopleura elegantissima is around 1.2 to 2.5 meters, a fact that will be very useful when making conjectures about its niche. The drop of abundance at 2.1 is only a local anomaly. At high heights (3.9, 3.6, etc.) there were no organisms present while at low heights there were some (however not very many).
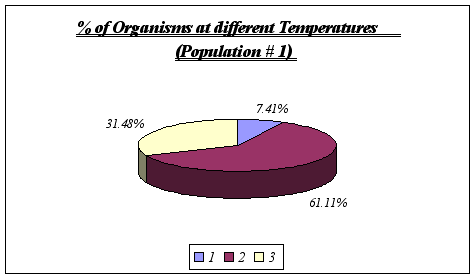
Despite the fact that a correlation could not be found between rock temperature and elevation or tidal height, an important feature was discovered, this being the relationship involving surface rock temperature and abundance. It was discovered that Anthopleura elegantissima prefers a rock temperature of 11, 12 or 13 because of the fact that these were the only temperatures found on the rocks (higher elevations were found to be warmer and A. elegantissima apparently does not favor such conditions).
Population # 2:
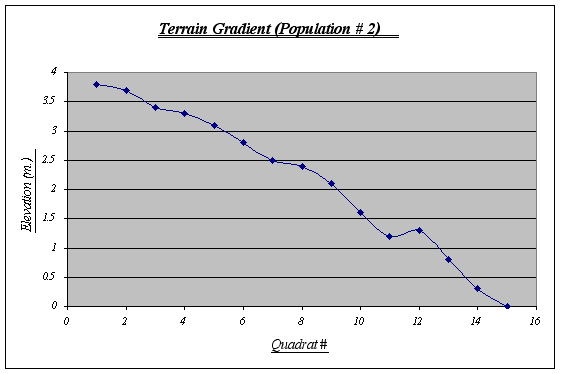
This gradient features a large tide pool at 1.25 meters level. In it, a whole new ecosystem is found due to different climatic conditions, therefore, it may create some distortions with the number of Anthopleura elegantissima expected. A steep fall at the end should also be noted as a probable cause for the distribution of the species.
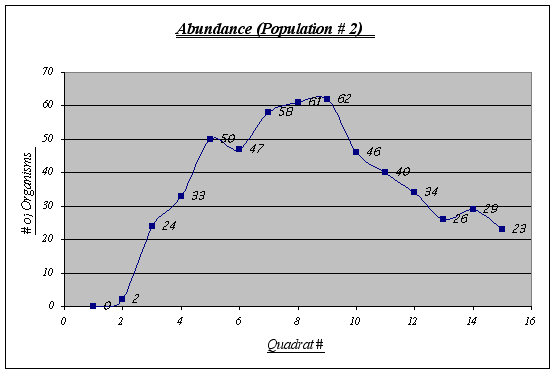 Figure 10.
Figure 10.In this population some irregularities are shown. Quadrats 6 and 13, for example, are not quite as expected. The virtual absence of organisms in quadrat 1 and 2 has to be considered as well. Overall, an inverted u-shaped curve could be distinguished.
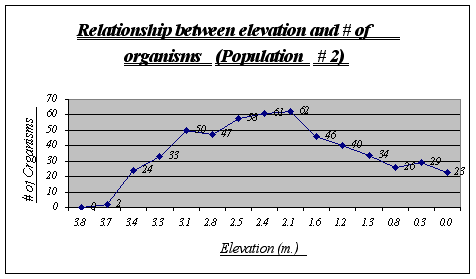
Again, the same trend as in population 1 is presented. A major distribution is seen at 2.1 to 2.8 meters and none or very few organisms were found at high elevations. On the other hand, a large number of the species was encountered at very low elevations
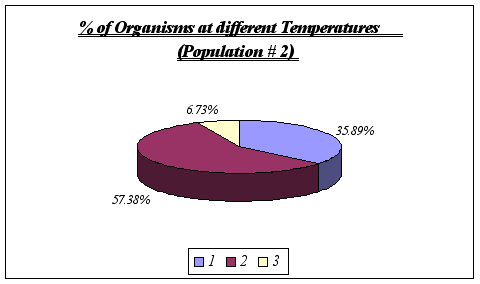
These results reinforce the idea that Anthopleura elegantissima prefer a range of temperatures of 11-13ƒ C. No further analysis could be made since it is unlikely that a distinction could be drawn using greater detail such as degree by degree. On the other hand, at temperatures significantly different from the range above, the species will not be found.
?
Population # 3:
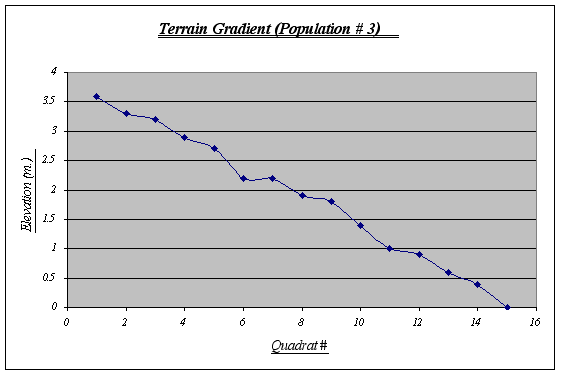
This gradient was the most irregular of the three, featuring ups and downs from the first to the last quadrat. A fact that seems rather curious is that on top of this strip sea lions lie down to rest quite frequently whereas this does not happen in the other two strips, probably because this gradient starts from a very plain, big rock. Kelp beds are observed floating on water and algae is seen at higher elevations.
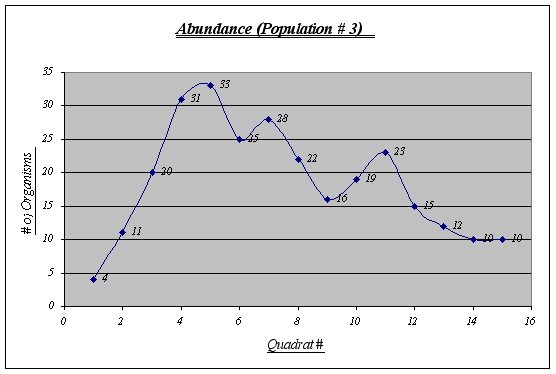
Although this graph does not show a perfect inverted u form, a general trend is seen. This population is the less abundant of the three and shows some irregularities in the middle quadrats.
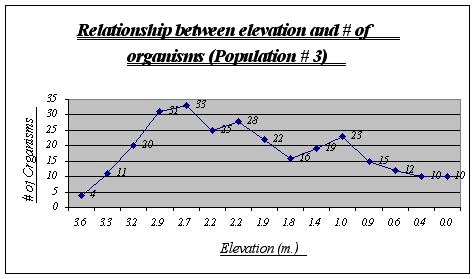
Apparently, more organisms prefer a range of 2.9 to 2.2 meters in this gradient. Some animals were found at 0.0 meters but not many were seen at higher heights. Two major drops, at 2.2 and 1.8 meters, can be explained due to overpopulation of other species in those two tide pools.
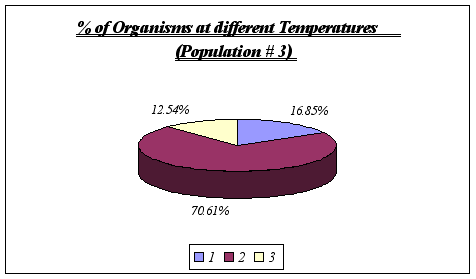
Once again, no other temperature ranges were found, leading to the conclusion that Anthopleura elegantissima does prefer temperatures of 11deg C to 13deg C.
Interpretation and Implications of the Findings: After looking at the graphs, general descriptions of the ecological niche of A. elegantissima could be made:
![]() ideal temperature for the species is a range of 11-13ƒ C because at higher or lower temperatures, the number of organisms decrease significantly. The null hypothesis is then rejected.
ideal temperature for the species is a range of 11-13ƒ C because at higher or lower temperatures, the number of organisms decrease significantly. The null hypothesis is then rejected.
![]() Idyllic elevation goes from 1.5 to 2.8 meters. Once again, the null hypothesis is rejected.
Idyllic elevation goes from 1.5 to 2.8 meters. Once again, the null hypothesis is rejected.
Since time underwater is a function of elevation, it was not considered on graphs. However, the preferable time underwater for Anthopleura elegantissima was found to be 5 to 15 hours approximately (out of 18 hs.)
A clear description of the ecological niche dimensions is observed in the graphs below. The larger bubbles represent the portion of the space that the species prefers:
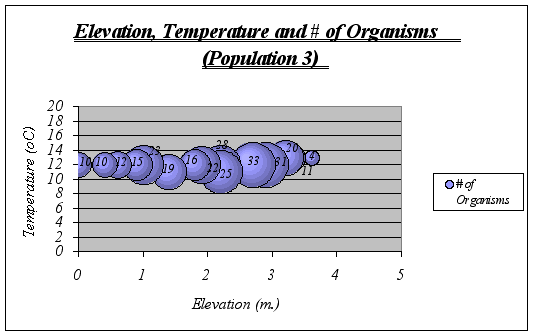 Figure 19.
Figure 19.Recommendations: If a thorough understanding of the ecological niche of Anthopleura elegantissima is desired, these and more variables should be tested to obtain a more profound and detailed approximation. Also, by determining ecological niches of other species such as Anthopleura xanthogrammica and comparing them, it is possible to predict niche overlapping, which is very likely to lead to a constant competition and aggressive behavior of the species. Moreover, it could be used to predict changes in the ecosystem if introduced species are brought. The documentation of data like this provides an invaluable record for establishing baseline distributions of organisms. Scientists are often required to monitor anthropogenic changes in sensitive marine environments. Similar niche patterns could be done on the other key invertebrates of the inter tidal and sub-tidal zone at Race Rocks, for example black leather chitons, limpets, abalone and various species of algae.
“Column green to white; tubercles usually in distinct longitudinal rows; tentacles with pink tips; height up to about 5 cm; often in aggregating masses, and frequently buried by sand covering rocks to which they are attached ‡ Anthopleura elegantissima. ~~ Column green or olive green; tubercles usually not in distinct longitudinal rows; tentacles uniform in color and not pink-tipped; height regularly exceeding 5 cm; solitary and not often buried in sand ‡ Anthopleura xanthogrammica.”
![]() Carefoot, T. 1977. “Pacific seashores.” J.J. Douglas, Vancouver, BC, Canada.?
Carefoot, T. 1977. “Pacific seashores.” J.J. Douglas, Vancouver, BC, Canada.?
![]() Colinvaux, P. 1986. “Ecology.” John Wiley & Sons, United States of America.
Colinvaux, P. 1986. “Ecology.” John Wiley & Sons, United States of America.
![]() Francis, L. 1973. “Clone specific segregation in the sea anemone Anthopleura elegantissima.” Biological bulletin. 144, 64-72.
Francis, L. 1973. “Clone specific segregation in the sea anemone Anthopleura elegantissima.” Biological bulletin. 144, 64-72.
![]() Francis, L. 1973. “Intraspecific and its effects on the distribution of Anthopleura elegantissima and some related sea anemones.” Biological bulletin. 144, 73-92.
Francis, L. 1973. “Intraspecific and its effects on the distribution of Anthopleura elegantissima and some related sea anemones.” Biological bulletin. 144, 73-92.
![]() Kozloff, E.N. 1974. “Keys to marine invertebrates of Puget Sound, the San Juan Archipelago, and adjacent regions.” University of Washington Press, Washington State.
Kozloff, E.N. 1974. “Keys to marine invertebrates of Puget Sound, the San Juan Archipelago, and adjacent regions.” University of Washington Press, Washington State.
![]() Kozloff, E.N. 1993. “Seashore life of the Northern Pacific Coast.” University of Washington Press, Seattle, Washington State.
Kozloff, E.N. 1993. “Seashore life of the Northern Pacific Coast.” University of Washington Press, Seattle, Washington State.
![]() ?Kucera, C. L. 1978. “The challenge of ecology.” The C. V. Mosby Company, Saint Louis.
?Kucera, C. L. 1978. “The challenge of ecology.” The C. V. Mosby Company, Saint Louis.
![]() Lewis, J.P. 1995. “La biosfera y sus ecosistemas: una introducción a la ecología.” Ecosur, Rosario, Argentina.
Lewis, J.P. 1995. “La biosfera y sus ecosistemas: una introducción a la ecología.” Ecosur, Rosario, Argentina.
![]() Odum, E. P. 1963. “Ecology.” Holt, Rinehart and Winston, United State of America.
Odum, E. P. 1963. “Ecology.” Holt, Rinehart and Winston, United State of America.
![]() Odum, E.P. 1989. “Ecology and our endangered life-support systems.” Sinauer, Sunderland, Massachusetts.
Odum, E.P. 1989. “Ecology and our endangered life-support systems.” Sinauer, Sunderland, Massachusetts.
![]() Pianka, E. R. 1986. “Ecology and natural history of desert lizards.” Princeton University Press, Princeton, NJ.
Pianka, E. R. 1986. “Ecology and natural history of desert lizards.” Princeton University Press, Princeton, NJ.
![]() Smith, R.I., and Carlton, J.T. 1975. “Intertidal invertebrates of the Central California Coast.” University of California Press, Los Angeles, California.
Smith, R.I., and Carlton, J.T. 1975. “Intertidal invertebrates of the Central California Coast.” University of California Press, Los Angeles, California.
![]() Zahid, M. 1987. “Distribution of Anthopleura elegantissima.” Extended Essay for the International Baccalaureate.
Zahid, M. 1987. “Distribution of Anthopleura elegantissima.” Extended Essay for the International Baccalaureate.
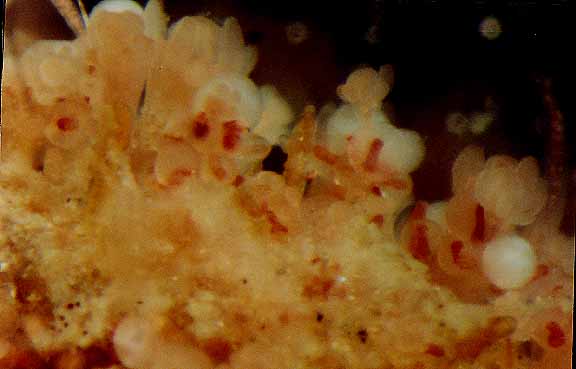
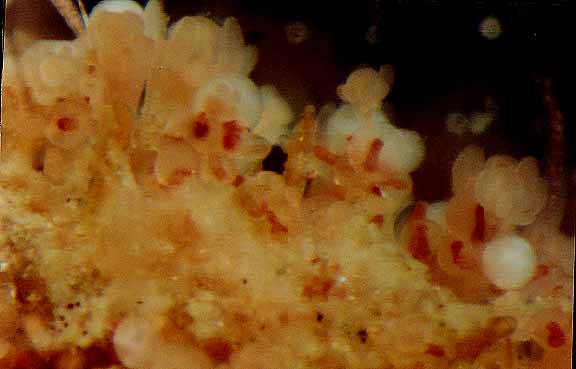
Hydractinia laevispina, (hydroid) Race Rocks taxonomy
This set of photomicrographs by Dr. Anita Brinckmann Voss shows a colony growing on a frond of Aglaophenia sp.
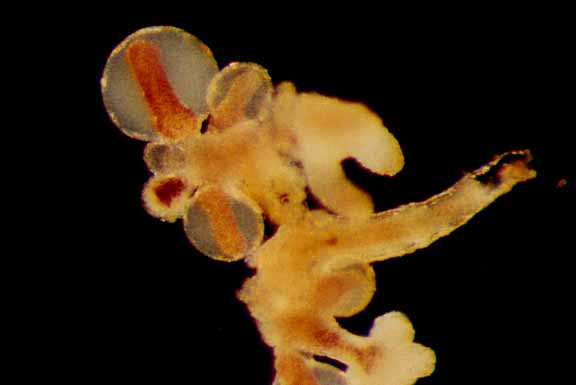
see this link for other hydroids: https://www.racerocks.ca/tag/hydroid/
Species recognized by World Register of Marine Species (WoRMS):
Biota
Animalia
Cnidaria
Hydrozoa
Hydroidolina
Anthoathecata
Filifera
Hydractiniidae L. Aggasiz, 1862
Hydractinia Van Beneden, 1841
Hydractinia laevispina Fraser, 1922
Return to the Race Rocks taxonomy and photo gallery
Clytia sp. hydroid: Photomicrography of Dr. Anita Brinckmann-Voss, The Race Rocks Taxonomy
- Clytia sp.colony with gonothek with medusae buds and enlarged hydranth. Found at Race Rocks, low intertidal.
- Enlarged hydranth of Clytia sp
| Domain | Eukarya |
| Kingdom | Animalia |
| Phylum | Cnidaria |
| Class | Hydrozoa |
| Order | Leptothecatae |
| Family | Campanulariidae |
| Genus | Clytia |
| Species | sp. |
This file is provided as part of a collaborative effort by Lester B. Pearson College and local scientists. Copyrighted 1999–All Images on this page are the property of Dr. Anita Brinckmann-Voss. They can not be used or modified without her written permission.
see this link for other hydroids: https://www.racerocks.ca/tag/hydroid/
Intertidal Invertebrates on the West shore of Great Race Rocks
 The intertidal zone on the West side of Great Race Rocks as viewed and photographed on June 12, 1999 at a minus 0.1 tide. The predominant macroalgae is Hedophylum sp. although immature bull kelp (Nereocystis luetkeana) is also anchored in this zone close to the shore.
The intertidal zone on the West side of Great Race Rocks as viewed and photographed on June 12, 1999 at a minus 0.1 tide. The predominant macroalgae is Hedophylum sp. although immature bull kelp (Nereocystis luetkeana) is also anchored in this zone close to the shore.  The small island on the North West corner is completely exposed at low tide but submerged at high tide. It contains a rich assortment of hydroids as well as other invertebrates where Dr. Anita Brinckmann-Voss has collected specimens at the zero tidal level.
The small island on the North West corner is completely exposed at low tide but submerged at high tide. It contains a rich assortment of hydroids as well as other invertebrates where Dr. Anita Brinckmann-Voss has collected specimens at the zero tidal level.
Small pink dots of a Melobesia mediocris, a calcareous pink encrusting algae which grows as an epiphyte on the leaves of surf grass.
 Just below the green fringe of surf grass, Phyllospadix scouleri, pink hydrocorals and other hydroid survive the current and wave swept zone.
Just below the green fringe of surf grass, Phyllospadix scouleri, pink hydrocorals and other hydroid survive the current and wave swept zone.
|
|||||||||||||||||||||||||||||
Color Polymorphism in the Intertidal Snail Littorina sitkana at Race Rocks
Patterns of Color Polymorphism in the Intertidal Snail Littorina sitkana in the Race Rocks Marine Protected Area.
- Figure 1 In Fig. 1 the snails were purposely placed on the white quartz substrate to show the contrast between a shell of color 27 ( white ) and some of colors 1 – 10 ( Black to grey ).
- Figure 2 The same process was repeated in Fig. 2 below only on black, basaltic substrate adjacent in the same tidepool. (Note three black snails (color 1-10) in lower left hand corner.)
- In Figure 3. Several colors of snail can be seen grazing on the golden diatoms in Pool 4 in the spring of 1998.
Extended Essay done by: Giovanni Rosso, Lester Pearson College, 1998 .
The complete version of the research is available in the Library at the college.
Abstract:
As with most intertidal gastropods, Littorina sitkana shows remarkable variations in shell color. This occurs both in microhabitats which are exposed or sheltered from wave action. There seemed to be a close link between the shell coloration of the periwinkle and the color of the background substrate. Field work was carried out on the Race Rocks Marine Protected Area in order to investigate patterns of color polymorphism. Evidence from previous studies was used to support interpretations and understand certain behaviors.
The results showed that in the study site there was a very strong relation between the shades of the shells and the colors of the rocks. Light colored shells stayed on light shaded rocks and vice versa. An interesting pattern was noticed with the white morphs. These were rare along the coast
(only 2%), but were present in relatively high numbers in tidepools of white quartz. From previous experience (Ron J.Etter,1988), these morphs seem to have developed as evolutionary response a higher resistance to physiological stress from drastic temperature changes between tides. Some results showed that the white morph is present in an unexpectedly high percentage at the juvenile stage, but then their number decreases dramatically. As in Etter’s study more research needs to be made on the role visual predators have in this phenomenon.
ROSSO, Giovanni Edoardo 0034 -083
Patterns of Color Polymorphism in the Intertidal Snail Littorina littorea at
the Race Rocks Marine Protected Area.
AN EXTENDED ESSAY PREPARED FOR THE INTERNATIONAL BACCALAUREATE
Candidate number: 0034 – 083 February 1999
Name: Rosso, Giovanni Edoardo
Best language: Italian
School: Lester B. Pearson College of the Pacific
Subject: Environmental Systems
Supervisor: Mr. Garry Fletcher
Table of contents:
Abstract ————————————————————— 3
Introduction ———————————————————- 4
Materials and methods ———————————————- 5
Data analysis ———————————————————- 7
Conclusion ———————————————————– 12
Observations ——————————————————— 13
Evaluation ———————————————————— 16
Suggestions for further studies ———————————— 16
Acknowledgments ——- ——————————————- 18
Literature cited —————————————————— 18
Appendix ————————————————————- 19
2
Abstract:
As most intertidal gastropods, the Littorina littorea shows remarkable variation in shell color. This occurs in both microhabitats that are exposed or sheltered from wave action. There appeared to be a close link between the shell coloration of the periwinkle and the color of the background surface. Fieldwork was carried out at the Race Rocks Marine Protected Area in order to investigate patterns of color polymorphism. Evidence from previous studies was also taken into account to better support interpretations and understand certain behaviors.
The results showed that in the study site there was a very strong relation between the color of the shells and the color of the rocks. Light colored shells lived on light shaded rocks and vice versa. An interesting pattern was noticed on the white morphs. These were rare along the coast (Only 2%), but were present in relatively high numbers in tidepools set in white quartz. From previous experience (Ron J Etter, 1988 ), these morphs seem to have developed, as an evolutionary response, a higher resistance to physiological stress from drastic temperature changes between tides. Some results showed that the white morph is present in an unexpectedly high percentage at the juvenile stage, but then their number decreases dramatically with age. As in Etter’s study, more research needs to be done on the role of visual predators in this phenomenon.
3
Introduction:
There is strong evidence to prove that intertidal gastropods are highly polymorphic for shell coloration (Ron J Etter, 1987). Even within a single species it is not uncommon to find considerable shell color variation in a single trait (Laurie Burham, 1988 ). In the genus Littorina the color of the shell often appears to be parallel to the one of the background (Heller, 1975; Smith, 1976; Reimehen, 1979; Hughes and Mather, 1986 ). Nevertheless the causes and patterns of color polymorphism. in intertidal gastropods are still a fairly unexplored field. Many paths have been undertaken to make some light upon these obscure areas. The most common interpretation was always the presence of visual predators (Ron J Etter, 1987) like birds and fish. Others investigated on the effects of the shells diets. But more recent studies ( Rowland, 1976; Ossborne, 1977; Berry, 1983 ; Etter, unpubl. ) have shown that diet virtually does not affect the shell coloration, although the intensity of pigmentation might be slightly altered. Finally, physiological stress has been introduced as a possible cause color polymorphism. A very interesting study, made by Ron J. Etter on the intertidal snail Nucella Lapillus, shows how the white coloration suffers much less from temperature variations in dry micro habitats as opposed to the brown morphs. With his work he gave some revolutionary insights on the distribution of the shells according to their color.
In my fieldwork I chose to disprove the null hypothesis that there is no link between the color of the periwinkle and the color of the substrate it is living on. In order to do this I sampled a great quantity of empty shells and scaled their color from I to 27. 1 then chose five rocky coastal areas, each of a different shade. I analyzed the color of the live shells on each of the chosen rocks, scaled them according to their color and then graphed the results. I also observed the young shells in the inside of barnacles and took notice of their color frequencies in relation to their quantity. I ended my study looking in some tide pools and recording new surprising results. I concluded that:
I roughly calculated that between one station and the other there was a change in tide level of 13 cm. I therefore kept this in account and lowered the quadrat accordingly into the water.
Data analysis-
Rock – 1 (Black)
The rock contained a creek were I noticed a very high density of periwinkles in a very limited area. In the inside of the creek they were almost piled and glued on top of each other. With the help of a pen I extracted them and laid them on a white sheet of paper. Once I accomplished the process of identification I put them back. I noticed that the bigger shells (10 to 14 mm wide) were located on top of the smaller ones (3 to 6 mm wide). This made me think that the bigger ones wanted to protect the smaller ones from swells and predators. It actually does work as a protection system, but it surely is not because of the kind nature of periwinkles. It is obviously a matter of physical size.
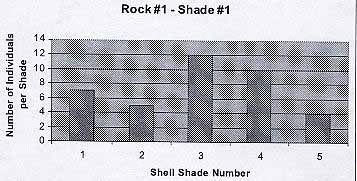
Rock #1 -Shade #1
From the graph we see that the black rock hosted the darkest shades, from 1 to 5. The average number of individuals per shade is 7.6. The average shell color is 3.
7
Rock # 2- Dark grey
As opposed to the previous case the surface of rock # 2 was rather flat. Population was regularly distributed. All shells seemed to be above 5 mm in width. Here I had the opportunity to understand the great resistance that periwinkles have to salinity changes. In fact some of the shells were located under the flow of a fresh water pipe. It might have been a coincidence but these shells were slightly bigger (7 to 12mm wide).
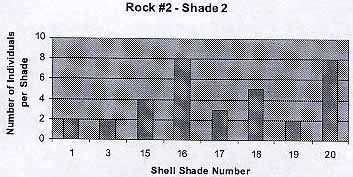
Rock #2 – Shade 2
The graph shows that there are some exceptions (Color 1, 3) to the trend that has been shown in the previous graph. I guessed that these are the cases of lucky shells that have not jet been seen by birds or fish. The average number of individuals per shade is 4.25 . The average shell color is 13.6 .
Rock – 3 (Brownish red)
The reddish color of the rock came from many small algae that covered its surface. I did not notice any irregular patterns in distribution. The shells seemed to be above 5 mm in width.
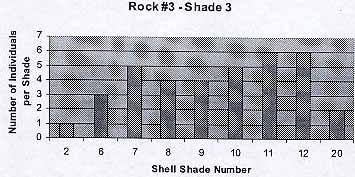
8
The background color was parallel to the shade of the periwinkles. Color 1 and 20 appear to be exceptions: only three individuals in total. The average number of individuals per shade is 4.4 . The average shell color is 9.4.
Rock – 4 (Light brown)
The surface of the rock was very irregular.. Some areas were covered with dead barnacles ( Balanus sp. ). I noticed that here the shells were smaller in size and they tended to be gathered around the barnacles. Nevertheless I repeated the process.
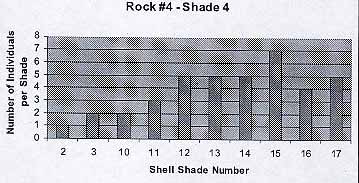
Rock #4 -Shade 4
9
The population reflects the previous trends. The average number of individuals per shade is 3.8. The average shell color is 11.3.
Rock – 5 (White rock with dark patches)
This rock was one of the most interesting ones. In fact, the two different shades of the rock gave place to a particular phenomenon that clearly disproved the null hypothesis. I tried to be as precise as I could in distinguishing the shells on the white and dark spots. I noticed the net distinction between the color polymorphism on the two areas.
Rock #5 -Shade 5
In the light patches the average number of individuals was 4.3. The average shell color was 23. In the dark areas the average number of individuals was 4.8. The average shell color was 3.
If the color of the shell would be directly proportional to the one of the rock, the average shell colors would be:
Ideal Model
10
Rock 1
| 3 | |
| Rock 2 | 13 |
| Rock 3 | 8 |
| Rock 4 | 18.5 |
| Rock 5 | 24.5 / 3 |
Actual Model
In the actual experiment the averages were:
Rock 1
| 7.6 | |
| Rock 2 | 13.6 |
| Rock 3 | 9.4 |
| Rock 4 | 11.3 |
| Rock 5 | 23 / 4.8 |
I assume that the dark grey rock is actually lighter than the brownish red
one. If we observe the results we understand that that:
| Rock Number Actual Shade |
Ideal One | Error | |
| 1 | 7.6 | 3 | 4.6 |
| 2 | 13.6 | 13 | 0.6 |
| 3 | 9.4 | 8 | 1.4 |
| 4 | 11.3 | 18.5 | 7.2 |
| 5 | 23 / 4.8 | 24.5 / 3 | 1 / 1.8 |
Considering that a minority of the shell color numbers was far away from the average: the average error is of 2.8. This means that on average the actual color was 2.8 units away from the ideal one, therefore disproving the null hypothesis. (Chi square test was used to verify the results.)
11
Conclusions:
The data analysis clearly shows that in the Race Rocks area there is a very strong relation between the color of the shell and the color of the background they are standing on. The shells with light shades are found on light colored rocks. The same relation is true also for the opposite extreme case were we find black shells on black rocks.
I feel that the model we can create from this experience is relevant above all because the consequences of human presence are reduced to very low levels. In fact, I have been operating in a Marine Protected Area were not many people go. The area is relatively free both from water and air pollution. The only predators are the natural ones. Besides this, the ecosystem is intact and the populations of all the organisms are at almost climax level. The amount of visual predators includes crabs, sea gulls, black oystercatchers, pigeon guillmonts, otters and fish.
From the observations made (p. 13, second part) on the entirely white morphs, we may deduce that there is a strong link between what Ron J. Etter found out on the Nucella lapillus and the Littorina littorea. Putting the pieces of the puzzle together we notice that the distribution of periwinkles is obviously affected by numerous reasons. There seems to be a wide color gap between the shades 1 to 26 and 27. The first twenty-six, when wet, are not very different from each other. The white morph instead is clearly identifiable both when it is wet or dry. If we keep in account that the vast majority of the coastal area on Race Rocks is dark, obviously it will be easier to for shells 1 to 26 hide. The white shells instead have such a great disadvantage that only 2% survive. Keeping in account Etter’s results we may conclude that, excluding a minority of extraordinary circumstances, all these deaths are caused by predators. In fact, when the juvenile periwinkles leave the barnacles, their shell is still soft. Now, if the white periwinkles are born near an area of white surface, then their chances of being seen decrease and actual groupings of white shells may be noticed. The color of their shell also allows them to bare physiological stress much better than the darker shades. The stress comes from the drastic changes in temperature between tide variations. In the case of the Nucella lapillus, in Etter’s experiment, the white shells inhabited most of the sheltered areas and, as previously mentioned, dry areas. This could also apply to the Littorina littorea, but on the Race Rocks Island the sheltered areas are very few and the number of predators is high. The white quartz is the only substrate that can host them (once they leave the Balanus sp.). I feel that if the ocean conditions were not as rough and there would be fewer
12
predators, the white morphs would also be seen on the darker rocks. In the tide pool both the white morph and the dark one live together The mortality of the last though is obviously higher, both for predation and stress (Ron J Etter).
In the dark areas the presence of the white morph is almost nonexistent (2%). But the shells belonging to shades 1 to 26 are distributed according to a remarkable pattern. On light colored rocks we will find shells that belong to the high numbers. In the opposite case the same trend applies. – In the area I took in exam this close relation is probably emphasized by the high intensity of predation. The contrasts are easily spotted and eliminated. Therefore, in the absence of predators, I think that the darker shells would be able to live on any color surface. Of course the dark population would suffer more in the dry areas as opposed to the lower levels.
Observations:
As I was watching the newborn shells (about 1 mm wide) in the dead barnacles I found out that the presence of white shells is unexpectedly high at this stage. I tried counting them and recording the results. On average a dead 20-mm wide Balanus sp. holds between four and eight shells of Littorina littorea. I analyzed ten samples in two different areas and recorded the number of white juvenile shells:
Area
| Total number of shells | Number of white shells | |
| 1 | 5 | 2 |
| 6 | 2 | |
| 8 | 4 | |
| 4 | 3 | |
| 4 | 1 | |
| 5 | 3 | |
| 6 | 4 | |
| 7 | 3 | |
| 6 | 2 | |
| 8 | 5 | |
| 2 | 4 | 2 |
| 7 | 4 | |
| 8 | 5 | |
| 3 | 0 | |
| 5 | 3 | |
| 7 | 4 | |
| 8 | 4 | |
| 6 | 3 | |
| 4 | 3 | |
| 9 | 5 |
In the first area the average Balanus sp. held 5.9 periwinkles and 2.9
were white or very light colored. The percentage of white shell was of 49.
In the second area the average Balanus sp. held 6.1 periwinkles and
3.3 were white. The percentage of white shells was of 54.
The results show that on average 51.5% of the shells are white. If we
make an exception for the tidepools, the percentage of white shells present
on the protected coastal areas is 2 (This is an approximate calculation made
when collecting the dead samples and when counting the live ones). This
means that 49.5% are eaten or die before reaching a sufficient size to move
in an area where they would be protected by the background they are
standing on. According to the study made by Ron J. Etter on the intertidal
snail, Nucella lapillus, when the brown morphs and the white ones were put
on the same exposed coastal area, there were virtually no differences in the
mortality rates of the two. If we dare to make a parallel between the two
species, it would be therefore wrong to assume that the white morphs die
because of natural causes such as diseases or disadaptation. It is my opinion
that literally 49.5% of the white morphs is victims of visual predators
because they can easily be seen before reaching an area where they would
camouflage. In this case, I am not including tide pools with white bottom
and where the water is shallow. I am referring to the morph with shade 27,
which is not common along the coast probably because of the lack of
almost entirely white rocks.
On the other hand I mentioned tied pools because of a specific reason. In
fact, on the Southwestern part of the island there are six tide pools, each
with different depths and different consequent bottom coloration. During
the days of the experiment this area was inaccessible for the presence of
about 75 California sea lions and about 23 Stellar. Nevertheless, in previous
visits to the island for other reasons (the reserve is in fact managed by
Pearson College and is used for several academic programs, projects and
environmentally oriented diving) I had the opportunity to observe the
presence in tide pool – 4 of about 20 entirely white shells of Littorina littorea
standing on white quartz. This had originated a question that had long
14
remained without an answer. Why can the white periwinkles be found only in this tide pool (if we exclude the two- percent I was talking about before)? The most common explanation was based on the presence of certain minerals, difficult predation and a genetic mutation that occurred only there. To be honest, after coming across Ron J. Etters study on the Nucella Lapillus, it was hard for me not to relate the two cases.. In his study he states that the white morph heats up at a lower rate as opposed to the brown morph in shallow and protected areas. Observing a higher rate of mortality (not due to predation) in the brown morph, he deduced that the white morph had developed a better defense mechanism against physiological stress. It therefore has higher chances of survival in very shallow water or in those areas that remain exposed between tides for a long time. Although brown snails can avoid exposure to the sun by moving to more shaded and moist microenvironments, Etter thinks their greater susceptibility to stress nonetheless puts them at a disadvantage by limiting their foraging area and increasing the amount of time that they must spend in hiding- This in turn could lead to slower growth rates and reduced levels of fecundity (Laurie Burnham, Scientific American, September 1988 ). On the other hand this does not exclude the presence of natural predators, especially in young age.
If we compare these results to the observations made on Race Rocks we may find many points in common. Especially after I had a new confirmation. In fact, in a tide pool with difficult access in another part of the island I found a similar behavior. On a small area of white quartz I found five entirely white periwinkles. There is a big difference in size between the ones I found there and the population of tide pool – 4. The first ones were about 2-3 mm wide; the second ones were 6-12mm. This might be due to the fact that they were living in a creek of difficult access to most predators. Nevertheless the pattern fits: the white periwinkles are almost all found in areas of shallow water or that remain exposed for a long time between the action of tides. On the other hand these are the only areas were white quartz is found on the island. The observations made on the fieldwork make me almost certain that the reasons for the white morphs to be in the tide pools are an adaptation to physiological stress and a perfect camouflage. In Etters experiment most of the protected areas were inhabited by white morphs. On Race Rocks only two tide pools contained such organisms and in very low quantities. I think this can be explained by the combination of several factors. In the first place the ocean conditions around the island are
15
very rough and they make it hard for the shells to survive in all areas.. In the second place there is very limited quantities of white rock were the shells can camouflage. Finally the very high quantity of visual predators, both from the air and form the sea, make it very difficult for these shells to move around because they will immediately be seen.
Evaluation:
Due to the lack of hi-tech material I had to verify my observations with simple tools- This forced me to use other people’s previous studies (Ron J. Etter) to better understand what I saw. If I had disposed of an instrument to measure the internal temperature of the shells I could have repeated Etters experiment on the Littorina littorea.
My experiments allow the creation of a model that is true, as far as we know, only on the Race Rocks Marine Protected Area. Other generalizations should be verified. In order to obtain a more reliable model the experiment should be repeated over a longer period of time on a regular basis. The month of October is a period when there is a significant increase in predation also due to the fact that the colony of seagulls on the island is incremented by the newborn.
I chose a vast scale of shade variations in order to achieve more precise results. By doing this, it was hard for me to identify exactly to which number each shell belonged. Even though I tried my best I might have made some mistakes.
Knowing that there are significant differences in distribution between the exposed and the sheltered areas, each of the sites was not exposed to the same environmental conditions. Some were more exposed to currents than others.
Suggestions for further studies:
As I mentioned in the introduction the causes and patterns of color polymorphism in intertidal gastropods are a fairly unexplored field. There are therefore still many grey areas that need to be cleared.
The fieldwork I had the opportunity to make on Race Rocks allowed me to learned many things on these fascinating creatures, but posed also many questions to which I have no answer.
I was surprised when I found so many white periwinkles in the barnacles. It would be interesting to find out exactly what happens to them once they leave these shells:
Who exactly are their predators?
16
At which stage in growth does their shell become too hard to be digested?
How do they choose the areas where they stop?
Are there certain types of minerals that create better conditions for living? Is there a link at all?
What is the exact probability for a shell to be white at birth?
Is the gene universal or is it majority- present in certain areas?
How does the alimentation affect growth and reproduction rate?
The white shells are more tolerant to physiological stress, but does this affect the immunitary system? Which diseases are the most common?
The fieldwork I have done seems to apply for Race Rocks, but is it true also in other nearby areas? To what extent does the exposure to rough environmental conditions affect distribution? Since the tide pool was covered and surrounded by sea lions, it was obviously affected by their waste products. The population of periwinkles seems to be fairly stable? How tolerant are these shells to changes in pH? Is there a difference between the degree of tolerance of the dark and the white morphs?
17
Acknowledgments
I sincerely thank my supervisor, Mr. Garry Fletcher, for his encouragement, support, precious advise and constructive criticism. I am also very grateful to Mr. Mike and Miss. Carol Slater for hosting me on the island during the field work. I will never forget the delicious supper we had together on Thanksgiving Day. In the end, I would like to thank Mr. Chris Blondeau for his sincere interest and for bringing me at Race Rocks by boat.
Literature cited:
Laurie Burnaham, September 1988, The hard shell, pp.26-27, Scientific American.
Ron J. Etter, April 1987, Physiological stress and color polymorphism in the
intertidal snail Nucella Lapillus, Museum of comparative zoology, Harvard
University, Cambrige, MA 02138.
Jane M. Hughes and Peter B. Mather, December 1984, Evidence for predation as a
factor in determining shell colorfrequencies in a Mangrove snail Littorina Sp.,
School ofAustralian Environmental Studies, Griffith University, Nathan,
Queensland,Australia.
18
APPENDIX 1. Photographs of Littorina littorea
In Fig. 1 the snails were purposely placed on the white quartz substrate to show the contrast
between a shell of color 27 (white) and some of colors
1 – 10 ( Black to grey).
The same process was repeated in Fig. 2 below only on black, basaltic substrate adjacent in the
same tidepool. (Note three black snails (color 1-10) in lower left hand corner.)
Figure 1 Figure 2
19
Apendix 2. Photographs of the shell shades of Littorina littorea
There was a very significant difference in color between the dry and the wet shells. In the two pictures some of the shells had to be moved around in order to maintain the darker periwinkles before the lighter ones. For example, wet shell number 11 had to be moved to 9 on the dry scale
20
Appendix 3. Picture from Ron J. Etters fieldwork
Ron J. Etter noticed that in the Nucella Lapillus the white morph was more common in the sheltered areas. The brown one dominated in the areas of wave exposure. He concluded that the color of sea shells on the seashore may be an evolutionary response to physiological stress.
The color of seashells on the seashore may be an evolutionary response to physiological stress
Photographs of Littorina sitkana Figure 1
Figure 1
In Fig. 1 the snails were purposely placed on the white quartz substrate to show the contrast between a shell of color 27 ( white ) and some of colors 1 – 10 ( Black to grey ).
The same process was repeated in Fig. 2 below only on black, basaltic substrate adjacent in the same tidepool. (Note three black snails (color 1-10) in lower left hand corner.)
 In Figure 3. Several colors of snail can be seen grazing on the golden diatoms in Pool 4 in the spring of 1998.
In Figure 3. Several colors of snail can be seen grazing on the golden diatoms in Pool 4 in the spring of 1998.
Abalone tagging at Race Rocks with Pearson College Divers
In 1998, we began a long term research program, initiated by Dr. Scott Wallace, on the population dynamics of the Northern Abalone
(Haliotis kamtschatkana). For several years, the Pearson College divers monitored the population. In this video, Pearson College graduate Jim Palardy (PC yr.25) explains the process.
SPECIES LIST From: William Head, Rosedale Rocks, Race Rocks by Donna Gibbs
SPECIES LIST:
Compiled by:Donna Gibbs of the Vancouver Acquarium on dives made at Rosedale Rock, West Race Rocks and William Head in the summer of 1997. Groupings are made in Phylums or Divisions.
Dive 432 – Rosedale, Race Rocks – June 12, 1997
|
|||||||||||||
Dive 433 – Rosedale, Race Rocks – June 13, 1997 |
|||||||||||||
|
|||||||||||||
Dive 434 – West Race Rocks – June 13, 1997 |
|||||||||||||
|
|||||||||||||
Dive 431 – William Head, Vancouver Island – June 12, 1997 |
|||||||||||||
|