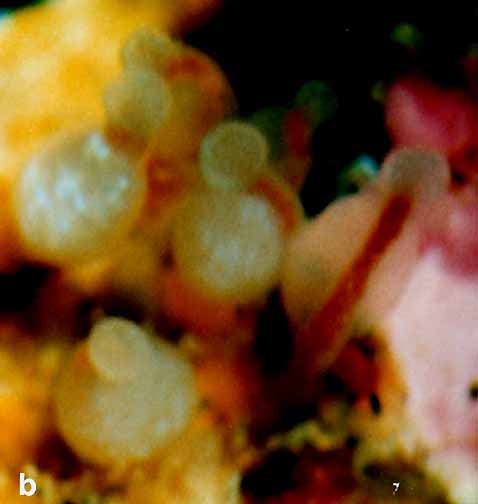Permission for reproduction of this paper has been granted by the Canadian Journal of Zoology and the Author. Color images have been taken by A.B.V. and D.M.L. and were added to this html document by G.Fletcher.
| p.401 , Vol 71, 1993 Rhysia fletcheri (Cnidaria, Hydrozoa, Rhysiidae), a new species of colonial hydroid from Vancouver Island (British Columbia, Canada) and the San Juan Archipelago (Washington, U.S.A.)A. BRINCKMANN-VOSS Department of lnvertebrate Zoology, Royal Ontario Museum, 100 Queen’s Park, Toronto, Ont., Canada M55 2C6And D. M. LICKEY AND C. E. MILLS, Friday Harbor laboratories, University of Washington, 620 University Road, Friday Harbor, WA 98250, U.S.A.Received February 28, 1992 Accepted September 17, 1992BRINCKMANN-VOSS, A., LICKEY, D. M., and MILLS, C. E. 1993. Rhysia fletcheri (Cnidaria, Hydrozoa, Rhysiidae), a new species of colonial hydroid from Vancouver Island (British Columbia, Canada) and the San Juan Archipelago (Washington, U.S.A.). Can. J. Zool. 71: 401-406. A new species of colonial athecate hydroid, Rhysia fletcheri, is described from Vancouver Island, British Columbia Canada, and from Friday Harbor, Washington, U.S.A. Its relationship to Rhysia autumnalis Brinckmann from the Mediterranean and Rhysia halecii (Hickson and Gravely) from the Antarctic and Japan is discussed. Rhysia fletcheri differs from Rhysia autumnalis and Rhysia halecii in the gastrozooid having distinctive cnidocyst clusters on its hypostome and few, thick tentacles. Most of its female gonozooids have no tentacles. Colonies of R. fletcheriare without dactylozooids. The majority of R. fletcheri colonies are found growing on large barnacles or among the hydrorhiza of large thecate hydrozoans. Rhysia fletcheri occurs in relatively sheltered waters of the San Juan Islands and on the exposed rocky coast of southern Vancouver Island.
On trouvera ici la description d’un nouvelle espece d’hydroide colonial sans theque. Rhysia fletcheri, trouvee dans l’ile de Vancouver en Colombie-Britannique, Canada, et a Friday Harbor, Washington, Etats-Unis. Sa relation avec Rhysia autumnalis Brinckmann en Medlterrannee et Rhysia halecii (Hickson and Gravely), de l’Antarctique et du Japon, fait l’objet d’une discussion. Rhysia fletcheri differe des deux autres especes par la presence chez le gastrozooide de faisceaux tres particuliers de cnidocystes sur l’ hypostome et de tentacules epais et peu nombreux. La plupart des gonozooides femelles sont depourvus de tentacules. Les colonies de R. fletcheri ne comportent pas de dactylozooides. La majorite des colonies de R. Fletcheri crois sent sur les grosses balanes ou parmi les hydrorhizes des gros hydrozoaires a theque. Rhysia Fletcheri se trouve dans les eaux relativement protegees des iles San Juan et sur la cote rocheuse exposee du sud de l’ile de Vancouver. [Traduit par la redaction.] |
Introduction:Colonies of a hydroid species belonging to the genus Rhysia Brinckmann, 1965 were collected off Friday Harbor in Washington State, U.S.A., from 1972 to 1992. They were found in tide pools at Race Rocks, British Columbia, Canada, and from adjacent coastal regions of Vancouver Island between 1986 and 1992. The species is referable to the hydrozoan family Rhysiidae, and to the genusRhysia, in having gonads within the body wall along one side of the gonozooid. However, it differs from previously described species of the genus in having cnidocysts arranged in clusters on the hypostome of the gastrozooid, and in having fewer and thicker tentacles on the gastrozooid, and no dactylozooids. The purpose of this paper is to provide a systematic and ecological account of Rhysia fletcheri sp.nov. The species is compared with Rhysia autumnalis Brinckmann, 1965, type species of the genus Rhysia, and with Stylactis halecii Hickson and Gravely, 1907. The latter species has lateral gonads, as doR. autumnalis and R. fletcheri sp.nov., and is assigned here to the genus Rhysia as well.ETYMOL0GY: Rhysia fletcheri is named for Garry Fletcher, senior biologist at Pearson College and voluntary warden of the Ecological Reserve of Race Rocks, British Columbia, Canada, who was instrumental in establishing Race Rocks as an Ecological Reserve in 1980.
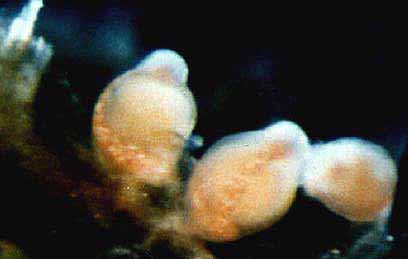
Material examined:  Growing on the valves of the barnacle Balanus nubilus , female and male colony .(.click on picture) . Top left, two females, below left gastrozooids: below right – male. Holotype: Friday Harbor, Washington, U.S.A., on Balanus nubilis attached to a tire on the side of floating docks at Friday Harbor Laboratories of the University of Washington, 0.5 m, 5 October 1984, female colony, National Museum of Natural History, Smithsonian Institution, Cat. No. USNM 73984. Paratypes: Race Rocks, British Columbia, Canada, on Semibalanus cariosus in tide pool, 0.5 m, 5 April 1990, male colony, Royal Ontario Museum Cat. No. ROMIZ B1164; Friday Harbor, Washington, on hydrorhiza of a thecate hydroid colony, 10-15 m, October 1972, female colony, Royal Ontario Museum Cat. No. ROMIZ B1165; Race Rocks, British Columbia, on Semibalanus cariosus in tide pool, 0.5 m, 15 June 1991, female and male colony, Royal British Columbia Museum Cat. No. RBCM 992-170-1. Further material is deposited in the Natural History Museum, London, England. Description: Hydroid colony stolonal, arising from a creeping and anastomosing hydrorhiza. Hydrorhiza thick (averaging 0.05 mm), covered with a very thin and often virtually invisible perisarc (Fig. 2a), giving rise to gastrozooids and gonozooids. Zooids inserting with hydrorhiza via a broad base and without a neck or stem (Figs. Ia, 2a); perisarcal collar absent around bases of zooids. Gastrozooids widely scattered, occurring singly or in a loose group. Gastrozooids extremely contractile, 0.3÷1.0 mm long, appearing columnar to barrel-shaped or like a contracted sea anemone if exposed to strong light (compare Figs. Ia and 4a). (Page 402) 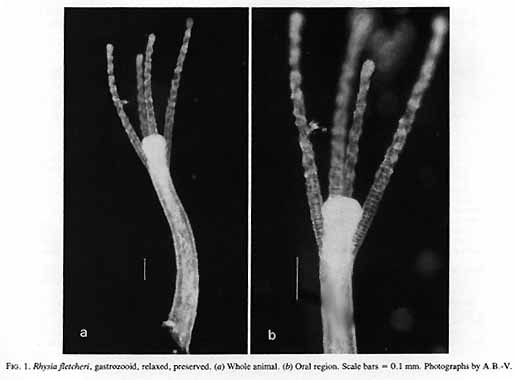 Figure 1. Rhysia fletcheri, gastrozooid, relaxed, preserved. (a) Whole animal, (b) oral region. Scale bars =0.1 mm. Gastrozocid tentacles 4 – 10, filiform, in a single whorl, 0.08 – 0.10 mm thick depending on the degree of contraction, each with more than 30 endodermal cells, cnidocysts arranged in a more or less distinct spiral (Fig. lb). Hypostome round, surrounded by a circle of 4 or 5 cnidocyst clusters that do not develop into tentacles (Figs. 2e, 2f, 4a). Gonozooids often separated from gastrozooids by several millimetres, occurring in dense clusters (Figs. 3, 4}. Gonads developing internally on one side of gonozooid, without a gonophore (Figs. 4b, 4c). Female gonozooids up to 1.1 mm high when mature (Figs. 3a÷3d); hypostome round, provided with a cap of cnidocysts, not divided into separate clusters as in gastrozooid; mouth lacking; tentacles typically lacking; in gastrozooid; mouth lacking; tentacles typically lacking; immature female gonozooids, at a stage not more than 115 the height of a mature gonozooid, being recognizable as such in showing an egg on one side. Male gonozooids develop 3 or 4 oral tentacles, which are shorter and thinner than those of gas- trozooids, each tentacle has up to 10 endodermal cells and bears cnidocysts at the tip only, some with thickened tips(Figs. 2c, 4b) because of the presence of larger numbers of cnidocysts (this varies among colonies); hypostome of males round, more conical than in females, provided with evenly distributed cnidocysts, unlike the cnidocyst clusters typical of gastrozooids; mouth lacking. Male gonozooids with mature gonads sometimes exceeding gastrozooids in length, reaching a maximum of 1.5 mm. Dactylozooids absent. Gastrozooids and gonozooids pink to orange, due to the colour of the endoderm; tentacles and hypostomes milky white; eggs and planulae peach coloured; male gonads milky white in early stages, iridescent in later stages.
|
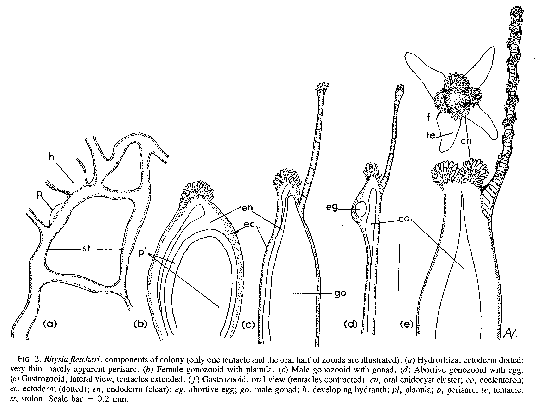
FIG. 2. Rhysia fletcheri, components of colony (only one tentacle and the oral half of zooids are illustrated). (a) Hydrorhiza, ectoderm dotted very thin, hardly apparent perisarc. (b) Female gonozooid with planula. (c) Male gonozooid with gonad. (d) Abortive gonozooid with egg. (e) Gastrozooid, lateral view, tentacles extended. (f) Gastrozooid, oral view (tentacles contracted) cn, oral cnidocyst cluster; co, coelenteron; ec, ectoderm (dotted); en, endoderm (clear); eg, abortive egg; go, male gonad; h, developing hydranth; pl, planula; p, perisarc; te, tentacle; st, stolon. Scale bar = 0.2 mm.
Development:: The single egg of the female is fertilized within the gonozooid. The location of sperm penetratlon is not known. Development of the embryo proceeds to the planula within the gonozooid. The planula is then released through rupture of the body wall. Before release of the planula the endoderm of the gonozooid may become reduced to a basal stump; however, the degree of reduction of the endoderm varies considerably. For histological details of development see Brinckmann’s(1965) description of the related species Rhysia autumnalis.The oval planula is about 0.8 mm long and does not swim. Instead it sank to the bottom and formed a stolon on the bottom of the glass bowl in the laboratory its development into a primary hydranth with four tentacles was seen once in the laboratory, but the colony did not develop further in non-aerated seawater. Field collected colonies that were kept in cirrculating seawater, however, did develop healthy fertile colonies.
Female gonozooids that develop a planula do not have tentacles . However, we occasionally observed very slim gonozooids carrying one small egg and three very slender tentacles (Fig4d). The single eggs in these zooids were pale yellow instead of the borange colour of typical female gonozooids, and a planula did not develop. We assume that fertilization of the egg did not occur in these atypical gonozooids, because they did not grow and eventually disintegrated. They were found in spring and early summer.
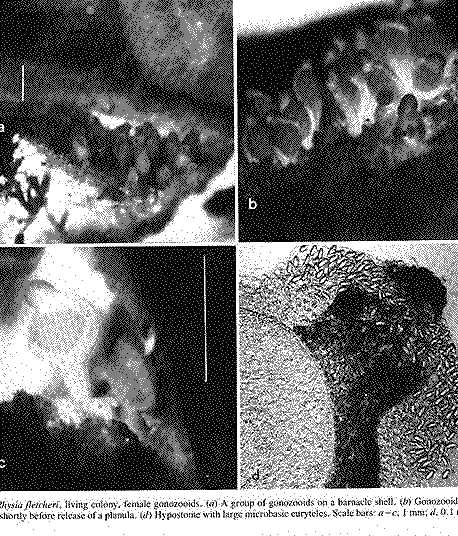
FIG. 3. Rhysia fletcheri, living colony, female gonozooids. (a) A group of gonozooids on a barnacle shell. (b) Gonozooids enlarged. lc) A gonozooid shortly before release of a planula. (d) Hypostome with large microbasic euryteles. Scale bars: a – c, I mm; d, 0.1 mm. Photographs by D.M.L. (Page 404.)
Ecological notes on the Genus Rhysia :
Rhysia fletcheri, described here, was found between January and October over a water temperature range of 6 – 14 degrees C, with fertile colonies from March to October, between 9 and 14C (no collections were made in November and December). The Mediterranean congener Rhysia autumnalis was collected between October and January, most fertile colonies occurring in November at a water temperature of about 14 – 15C. (Brinckmann 1965).Rhysia halecii, from the Antarctic, was found among a subglacial (no specific temperature was given) collection of hydroids from McMurdo Bay, Antarctica (Hickson and Gravely 1907).
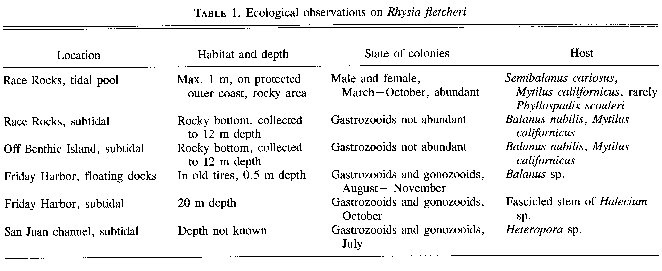
Colonies of Rhysia fletcheri were collected from floats (about 0.5 m deep) in a protected bay at Frlday Harbor, in strong-current channels of the San Juan Islands (to 20 m deep), and along the southwestern coast of Vancouver Island (to 12 m deep), or in tide pools (to 1 m deep). All collection sites were over rocky bottom (Table 1). Rhysia autumnalis was found in rocky areas of the southern part of the Gulf of Naples, off Sorrento, Italy (to 50 m deep) (Brinckmann 1965), and on a vertical rock wall off Protofino, Italy (7 m deep) (Boero and Fresi 1986). Rhysia halecii from McMurdo Bay in the Antarctic was collected at about 36 m depth (20 fathoms (Hickson and Gravely 1907) and Rhysia halecii (see Discussion) from Sagami Bay, Japan, at 5 – 9 m depth (Hirohito l988). There is no information on the substratum for R. halecii from the Antarctic or Japan.Colonies of the three described species of the genus Rhysia live mostly in association with other invertebrates. Rhysia fletcheri grows in the protected crevices of the shells of large barnacles or mussels or within the hydrorhizal system of the larger colonial hydroids, rarely on the leaf base of the surf-grass Phyllospadix scouleri. Rhysia autumnaliswas commonly found in association with the opisthobranch mollusc Vermetus sp., either living directly on its shell, or among the stolons of Eudendrium racemosum or the thalli of algae, which both grow on the shell of Vermetus. Boero and Fresi (1986) report R. autumnalis on algae. The hydroid identified as R. halecii (see Discussion) from Japan by Hirohito (1988) was found on the alga Acanthopeltis japonica.
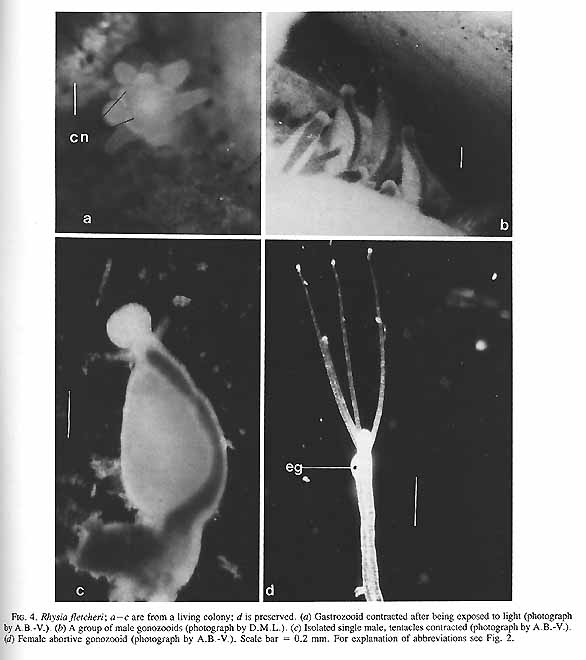
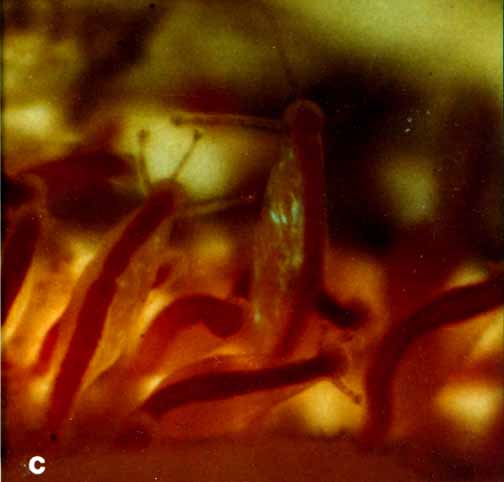
FIG. 4. Rhysia fletcheri; a÷c are from a living colony; d is preserved. (a) Gastrozooid contracted after being exposed to light (photograph by A.B.-V.). (b) A group of male gonozooids (photograph by D.M.L.). (c) Isolated single male, tentacles contracted (photograph by A.B.-V.). (d) Female abortive gonozooid (photograph by A.B.-V.). Scale bar = 0.2 mm. For explanation of abbreviations see Fig. 2. FIG. 4. Rhysia fletcheri; a÷c are from a living colony; d is preserved. (a) Gastrozooid contracted after being exposed to light (photograph by A.B.-V.). (b) A group of male gonozooids (photograph by D.M.L.). (c) Isolated single male, tentacles contracted (photograph by A.B.-V.). (d) Female abortive gonozooid (photograph by A.B.-V.). Scale bar = 0.2 mm. For explanation of abbreviations see Fig. 2. (Page406) FIG. 4. Rhysia fletcheri; a÷c are from a living colony; d is preserved. (a) Gastrozooid contracted after being exposed to light (photograph by A.B.-V.). (b) A group of male gonozooids (photograph by D.M.L.). (c) Isolated single male, tentacles contracted (photograph by A.B.-V.). (d) Female abortive gonozooid (photograph by A.B.-V.). Scale bar = 0.2 mm. For explanation of abbreviations see Fig. 2. (Page406) FIG. 4. Rhysia fletcheri; a÷c are from a living colony; d is preserved. (a) Gastrozooid contracted after being exposed to light (photograph by A.B.-V.). (b) A group of male gonozooids (photograph by D.M.L.). (c) Isolated single male, tentacles contracted (photograph by A.B.-V.). (d) Female abortive gonozooid (photograph by A.B.-V.). Scale bar = 0.2 mm. For explanation of abbreviations see Fig. 2. (Page406) FIG. 4. Rhysia fletcheri; a÷c are from a living colony; d is preserved. (a) Gastrozooid contracted after being exposed to light (photograph by A.B.-V.). (b) A group of male gonozooids (photograph by D.M.L.). (c) Isolated single male, tentacles contracted (photograph by A.B.-V.). (d) Female abortive gonozooid (photograph by A.B.-V.). Scale bar = 0.2 mm. For explanation of abbreviations see Fig. 2. (Page406)
Discussion:
Rhysia fletcheri can be distinguished from its congeners R. autumnalis Brinckmann and R. halecii (Hickson and Gravely) on the basis of morphological characters (Table 2). Meristic characters largely distinguish R. autumnalis and R. halecii. Furthermore, these species are also widely separated zoogeographically and occur under quite dissimilar environmental conditions. Unfortunately, the gonozooids and cnidocysts of the female were not described in the original description of R. halecii, and its relationships with the other species in the genus are difficult to determine at present. Hickson and Gravely (1907) originally referred R. haleciito the genus Stylactis (family Hydractiniidae). Hirohito (1988) claimed that the degree of reduction of the gonozooid did not justify the founding of a separate family, Rhysiidae, and genus, Rhysia, and declared Rhysia synonymous with Stylactis, in the family Hydractiniidae. However, the structure of the gonozooid, with its reduction of the gonophores to mere gonads, is very different from that of representatives of the Hydractiniidae, and we maintain that the family Rhysiidae, with its only genus at present, Rhysia, is valid (see also Brinckmann 1965; Bouillon 1978, 1985; Calder 1988; Petersen 1979; Werner 1984). Bouillon (1978) included the Rhysiidae in the now recognized superfamily Hydractinoidea Agassiz, 1862. As for the generic name Stylactis, it was shown to be a junior synonym of Hydractinia by Calder (1988). Stechow (1925) included Stylactis halecii in his genus Halerella Stechow, 1922, which has not been adopted as a valid name (Kramp 1932; Millard 1975; Bouillon 1985; Calder 1988).
Hirohito (1988) assigned hydroids found on algae from Japan to “Stylactis halecii.” Given differences between the Antarctic and Sagami Bay in terms of environmental factors such as temperature, it seems unlikely that the Japanese species of Rhysia is conspecific with the Antarctic R. halecii. The very dense network of the hydrorhiza, the absence of separate cnidocyst clusters on the hypostome of the gastrozooid, and the presence of dactylozooids distinguish the Japanese species from R. fletcheri. We were unable to obtain material of the Japanese Rhysia for comparison.
Cnidocysts of R. autumrlalis and R. fletcheri examined by us included desmonemes, and microbasic euryteles of both large and small size classes. Brinckmann (1965) was mistaken in referring to euryteles as stenoteles. The size and distribution of large and small microbasic euryteles on the hypostome and tentacles are similar in both species. Large microbasic euryteles in these hydroids were concentrated in the hypostome (Figs. 2h, 3d), and they were much more numerous in gastrozooids than in gonozocids. Small microbasic euryteles and desmonemes were observed on the tentacles. They were abundant on the tentacles of gastrozooids but scarce on the tentacles of gonozooids, being confined to the tips. Cnidocysts have not been described for R. haleci’ from the Antarctic, nor for the hydroid reported as that species from Japan.
Acknowledgements:
-
- We thank Dr. Dale Calder, Department of Invertebrate Zoology, Royal Ontario Museum, Toronto, for reviewing themanuscript and for many helpful suggestions. We are gratefuI to those who made the studies at Race Rocks possible, especially Garry Fletcher from Pearson College and Joan and Charles Redhead, former lighthouse keepers at Race Rocks. We also thank the Royal Ontario Museum and the Friday Harbor Laboratories for use of their equipment and space.
References Cited:
Boero, F., and Fresi, E. 1986. Zonation and evolution of a rocky bottom hydroid community. Pubbl. Stn. Zool. Napoli Sect. I Mar. Ecol. 7: 123 – 150.
Bouillon, J. 1978. Sur un nouveau genre et une nouvelle espece de Ptilocodiidae,Hydrichtelloides reticulata et al superfamille des Hydractinoidea (Hydroida-Athecata). Steenstrupia, 5(6): 53-67.
Bouillon, J. 1985. Essai de classification des Hydropolypes÷ Hydromeduses (Hydrozoa – Cnidaria). Indo-Malayan Zool. 1: 29 -243.
Brinckmann, A. 1965. The biology and development of Rhysia autumnalis gen.n., sp.n. (Anthomedusae/Athecatae, Rhysiidae fam.n.). Can. J. Zool. 43: 941-952.
Calder, D. 1988. Shallow-water hydroids of Bermuda. The Athecatae. Life Sciences Contrib. No. 148, Royal Ontario Museum, Toronto.
Hickson, J. S., and Gravely, F. H. 1907. Hydroid zoophytes. Natl. Antarct. Exp. Nat. Hist. 3: 1 – 34, Plates I÷4.
Hirohito, Emperor of Japan. 1988. The hydroids of Sagami Bay, Biological Laboratory, Imperial Household, Tokyo, Japan.
Kramp, P. 1932. The Godthaab Expedition 1928. Hydroids. Medd. Gronl. 79: 1-86.
Millard, N. A. H. 1975. Monograph on the Hydroida of southern Africa. Ann. S. Afr. Mus. 45.
Petersen, K. W. 1979. Development of coloniality in Hydrozoa. In Biology and systematics of colonial organisms. Syst. Assoc. Spec. Vol. 11: 105-139.
Stechow, E. 1925. Die Hydroiden von West- und Sudwestaustralien nach den Sammlungen von Prof. Dr. Michaelsen und Prof. Dr. Hartmeyer. Zool. Jb. Syst. Oekol. Geogr. Tiere, 50: 191-269.
Werner, B. 1984. Stamm Cnidaria, Nesseltiere. In Lehrbuch der speziellen Zoologie. Band I. Wirbellose Tiere. 2. Teil. Cnidaria. Gustav Fischer Verlag, Stuttgart, Germany. Pp.11-305.
insrert