METHODSand APPARATUS:
Part Ia:
The apparatus used to test for light versus shade preference was very similar to that used by Geiger and Holyoak (1996). It consisted of an opaque white simple flow tank 162 cm long by 74 cm wide by 15 cm deep, with a drain at one end of the table (on left in all figures) and a tube supplying water at the other end. One end of the tank was covered with an opaque black plastic sheet so that half of the tank received light from the overhead fluorescent light (40 W), and the other half of the tank was shaded. Thus, half of the tank was in darkness and half in light with the light-dark interface (border) aligned along the nudibranch’s longitudinal axis at the beginning of each test. The windows of the tank room were covered during the tests so that the only light entering the tank was from an overhead light suspended 1.5 m above the water surface. The water temperature was kept constant for ocean temperatures at that time of year, ranging between 8 and 12oC. 54 test runs of 40 minutes duration comprising 36 hours of experiment over two periods (May 17-19 and October 21-29, 2000) were completed. At the beginning of each test run, individuals of one or more species were placed in the tank with the light-dark interface running along their mid-sagittal planes. The distance between adjacent nudibranchs was standardised[2], and the direction they were facing as well as the side that was shaded was randomised to negate any genetic predispositions for turning left or right, as proposed by Geiger and Holyoak (1996). Time trials were 40 minutes long, and the position of each specimen was recorded at 5-minute intervals. This observation method is an improvement on the procedure of Geiger and Holyoak who used a nearly identical set up as previously described, but only recorded nudibranch positions at the end of the test. The Geiger and Holyoak (1996) method lead to inaccurate impressions because animal position during the course of the run was not considered. The procedure used in this experiment is an improvement as it determines not only the specimen’s endpoint, but also time ratios spent in either section of the tank and interactions between specimens. The other difference between the Geiger and Holyoak procedure and the one used in this study is that time trials in this experiment were shortened from 60 minutes to 40 minutes. It was observed after the first 4 test trials that, given the specimen’s measured speed and direction, under no circumstances would it turn and reach the shaded end of the tank in the following 20 minutes. In this way, more trials could be conducted and more descriptive data could be obtained per time spent. The simple flow tank was scrubbed and rinsed with fresh seawater after every test run. For Geiger and Holyoak’s complete method, see Appendix B.
Part Ib:
A modification was made to the experimental method for the last battery of tests[3]. Tests conducted in this phase of the experimentation were identical in setup to the previous ones, but only the initial shade or light preference was recorded. The experiment lasted only 5 minutes, allowing for more test runs to be made in a shorter period of time. This modification was made after the initial 54 tests of 40 minutes duration that consisted of 73 individual results. Of the 73 subjects tested, 98.6% did not change lighted as opposed to shaded condition preference during the test. In other words, 1.4% of subjects tested did change preference during the test runs (see Figure 5). Therefore it was hypothesised that results from this subsequent experimental phase would be accurate to ±1.4%.
Part II:
To investigate how water current strength affected the test run results, a slight modification to the procedure was made. In Part I of the experiment, shade preference was analysed at the midpoint between the drain and the seawater flow where current strength was minimal. In Part II, the same apparatus was used, but the nudibranch specimens were lined up 5 cm from the output of the seawater tube. Here currents were stronger than anywhere else in the tank. Another variation from the procedure of Part I is that specimen were only observed for 5 minutes. This short time was chosen because the initial direction of movement chosen by the specimens (towards/away from light or water current) was hypothesised to be more informative than specimen locations after 40 minutes time.
DATA COLLECTION:
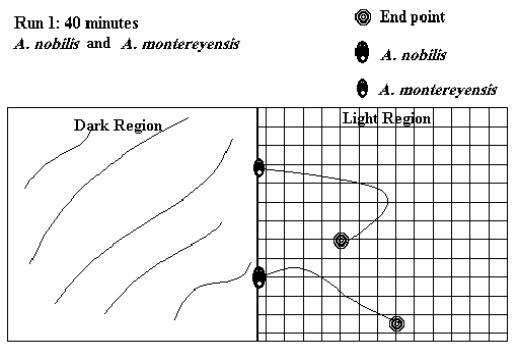
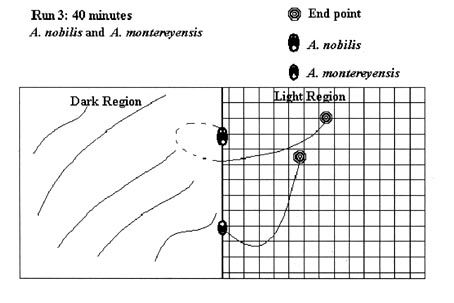
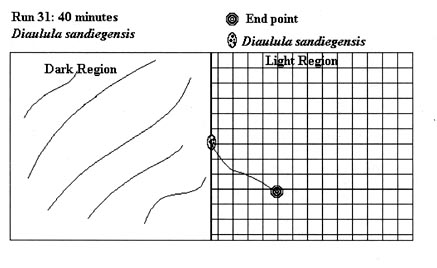
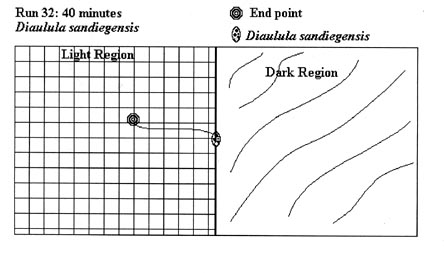
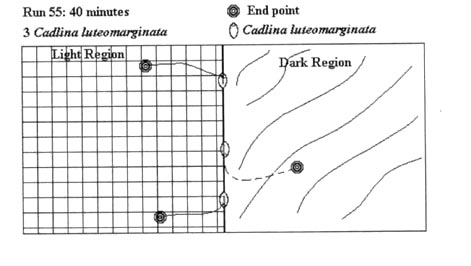
Since the test runs had either positive (light end) or negative (shaded end) results, and specimens moved only on a two-dimensional plane, not all test runs are graphically represented here. 54 illustrations would take an inordinate amount of space, so the following illustrative examples of test runs conducted during the experiment were chosen. These examples were chosen as they accurately represent the average test run. The actual test run number is indicated in the figure.
Table 1:
Preference for lighted versus shaded conditions by adults of five species of dorid nudibranchs based on the number of test specimens in defined areas at the end of 40-minute test runs. See Methods Part Ia.
No. in lighted No. in shaded Time Ratio Actual (min)
Species end of tank end of tank Light : Dark Light : Dark
A. montereyensis 13 2 13 : 2 520 : 80
A. nobilis 10 3 3 : 1[4] 390 : 130
D. sandiegensis 14 7 2 : 1 560 : 280
C. luteomarginata 10 8 5 : 4 400 : 320
A. hudsoni 5 1 5 : 1 200 : 40
Table 2:
Preference for unshaded versus shaded conditions by adults of five species of dorid nudibranchs based on initial condition preference by test specimens at the end of 5-minute test runs. See Methods Part Ib.
No. preferring No. preferring
Species lighted conditions shaded conditions
A. montereyensis 7 5
A. nobilis 9 3
D. sandiegensis 8 4
C. luteomarginata 16 20
A. hudsoni 10 2
Part Ia and Part Ib of experimentation clearly demonstrate that there was no marked shade preference expressed by any of the nudibranch species tested. In fact, with the exception of C. luteomarginata, all species showed a distinct preference for lighted conditions. The results for A. montereyensis and D. sandiegensis in this experiment are greatly different from those obtained by Geiger and Holyoak (1996), as shown in Figure 13.
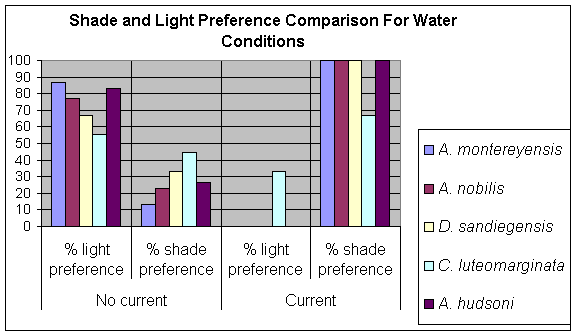
Table 3:
Preference for shaded versus lighted conditions by adults of five species of dorid nudibranchs Part II: When in Direct Current.[5]
Total
No. in light No. in shade No. in shade end
Species end of tank end of tank and into current
A. montereyensis 0 4 2
A. nobilis 0 4 2
D. sandiegensis 0 4 2
C. luteomarginata 2 4 3
A. hudsoni 0 4 2
DATA ANALYSIS:
Table 4:
Light versus Shade Preference of dorid nudibranchs: A comparison between this investigation’s results and those of Geiger and Holyoak (1996).
Murphy (2000) Geiger and Holyoak (1996)
% light % shade % light % shade
Species preference preference preference preference
A. montereyensis 86.7 13.3 8.3 91.7
A. nobilis 76.9 23.1 n/a n/a
D. sandiegensis 66.7 33.3 0 100
C. luteomarginata 55.6 44.4 n/a n/a
A. hudsoni 83.3 26.7 n/a n/a
Figure 13:
Light and Shade Preference of dorid nudibranchs, comparison between this investigation’s results with those of Geiger and Holyoak (1996)
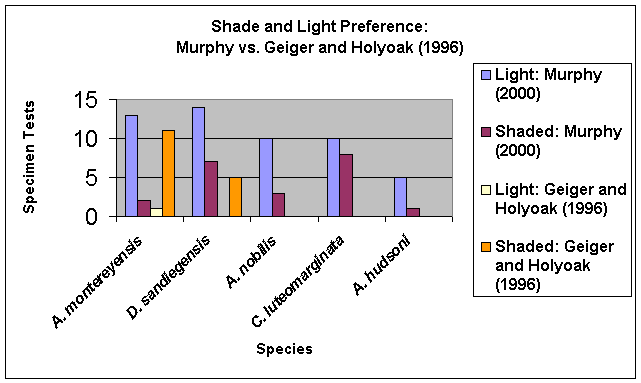
These illustrated results show the clear discrepancy between the results obtained in this investigation and those obtained by Geiger and Holyoak in their investigation.
Table 5:
Paired-t test analysis on effect of drain position for all nudibranch test runs. Data obtained from parts Ia and Ib have been presented separately to acknowledge the two methods of experimentation. The data is pooled together for calculations.
Part Ia: Light Shaded Part Ib: Light Shaded
Drain[6] 25 9 Drain 23 19
Not Drain 27 12 Not Drain 27 15
Paired t-test: t = 4.32 df = 3 P < 0.05 P = 0.023
n = 11
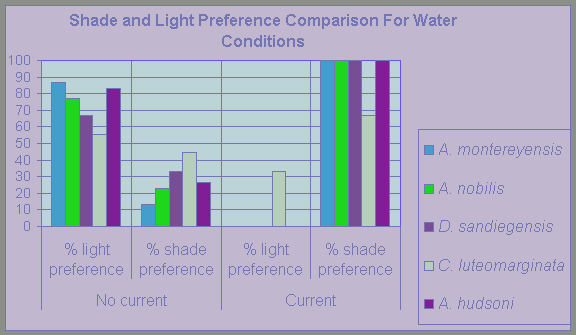
Figure 14
Comparison of light and shade preference by nudibranch species when tested in a tank with and without water current.
This data comes from Tables 1, 2 and 3.
EVALUATION:
None of the five species showed the shade preference as suggested by Geiger and Holyoak (1996). There could be several reasons why this is true. First, Geiger and Holyoak collected their specimens from the intertidal zone along the pilings of the institute where they were studying, an area similar to the tidal creek used by Biermann and her team. These conditions are specific to San Juan Island as it is generally accepted that neither A. nobilis nor A. montereyensis are found in the intertidal zone in many areas (Morris et al,, 1983; Werry, 1996). The specific conditions unique to Argyle Creek (48o31’ N, 123o01’ W) near where both Geiger and Holyoak, and Biermann et al. (1992) conducted their research are therefore important to note (Damkaer, et al., 1981). Notice (Figures 2 and 3) that Argyle Creek is 14’ north and 30’ east of Great Race Rock, well inside Puget Sound and sheltered from the powerful currents that are so influential at Race Rocks. The specimens collected at Great Race Rock were at a depth of no less than 6m. The massive shifts in current and oceanic swells (creating submarine surge) that occur frequently at Race Rocks are very hazardous for non-sessile marine life above this depth.
The data presented in Table 2 seems to suggest that current speed does have a noticeable impact on nudibranch shade preference. A. montereyensis for example shows a shade preference in calm waters of 14.3% in comparison to shade preference of 100% in waters with current (Figure 14). In the natural environment, direct shade as supplied in the laboratory comes from macroalgal shelter, generally of the Agarum fimbriatum and Laminaria sp. kelp species. These kelps have broad blades[7] that are capable of shielding the benthos[8] in the ecosystem from not only sunlight but from currents as well. This aspect of kelp-benthos interactions is of great importance in ecosystems like those of Race Rocks, where strong currents have the potential of pulling organisms off the bottom and depositing them in areas much less habitable for them (Hadfield, 1986). Finding refuge under macroalgal shelter may be a defence mechanism against such threats. This correlates to shade preference expressed during tests in Part II of experimentation (see Figure 14). Specimens moving to shaded conditions, even though it was against the current suggest that shade is associated with a protection from current force.
In Part II of experimentation, initial light versus shade preference was noted for the experiment. Tests in Part II were conducted immediately after the nudibranch had been physically removed from the bottom of the tank and placed in an area of strong current, relatively speaking. It was observed though, that over night, most (71.4%) individuals had migrated towards the seawater flow, even if they had shown a preference against it during experimentation. The initial reaction is interesting in that it reflects a behavioural mechanism (perhaps of a defensive nature), while final resting distribution after a disturbance reflects preference (see also Thorson, 1964).
Geiger and Holyoak postulated that the distribution of Haliclona sp. in shaded areas had an effect on nudibranch behaviour (1996). This argument is not convincing in that all nudibranch species studied have shown a preference for Halichondria panicea, as well as Haliclona sp. when feeding in the laboratory. No such shade-influenced dispersion of H. panicea has been reported, leading to the conclusion that dietary habits are not a factor influencing shade or light preference in this subtidal population of dorid nudibranchs (Elvin, 1976).
Another variance that may have influenced test results is the heterogeneity of runs. The tests conducted during this study contained multi-species as well as mono-species runs, unlike the solely homogeneous runs conducted by Geiger and Holyoak (1996). Poly-species runs showed that there was no preference for shaded conditions, but rather a preference for lighted ones, as did the mono-species runs. Here there is no correlation with aggregation of adults of the same species, often seen in nature, for the means of exchange of autosperm and allosperm and shade preference. It would be of interest to determine whether or not such aggregations of intertidal populations would produce a shade preference as noted by Geiger and Holyoak (1996).
There are several improvements that can be made to this experiment. The first deals with the population from which specimens were obtained. There has yet to have been an experiment where specimens from intertidal and subtidal populations were tested under the exact same circumstances. Though this investigation and Geiger and Holyoak’s 1996 paper have complimentary data, they do not allow for exact comparisons. Such a comparison would be crucial for evaluating the postulations made in both investigations.
The effects that drain position had in test runs was analysed by the Paired t-test (see Table 5). The probability (P) that drain position was influential upon the runs was less than 0.05. This is an accepted value for being negligibly influential, though not as low as the 0.01 accepted value for no influence. This statistical test showed that while the drain did have an effect on the tests, it was negligible and thus not a large limitation to this procedure (Vogel, et al., 1978).
Part Ib of the experiment was intended to increase the number of values on which statistical analysis could be performed in a shorter amount of time. Though this goal was achieved, the results differed noticeably from Part Ia. Values presented in Table 4 demonstrate the increased shade preference by the subjects for this portion of experimentation. A possible explanation for this occurrence is stress of the individuals. During Part Ia, subjects were handled for no more than 60 seconds and then observed stress free for 40 minutes. In Part Ib however, subjects were handled for the same amount of time, but were only given 5 minutes of rest. The fatigue and stress caused by this increase in handling and frequent repetition is quite possibly the reason for the increase in shade preference. This stress-induced shade preference supports the kelp-benthos interaction previously mentioned, in that shade is associated with an environment where a stress factor is less likely to occur. This method, while yielding results and being time effective, was shown to be less accurate than Part Ia.
Though Part II of this investigation dealt with current strength, an improvement on the Geiger and Holyoak (1996) procedure, it failed to collect quantitative data for water current strength. By gauging this variable and conducting test runs under more controlled circumstances where current strength could be monitored and controlled, more enlightening data could be collected on this subject.
One last improvement to this procedure would be to monitor these subtidal nudibranch populations in the laboratory and nature and to determine whether there is an exhibited shade preference for egg ribbon depository sites. The Friday Harbour population exhibited shade preference both for routine behaviours and for egg depositing (Biermann, et al., 1992; and Geiger and Holyoak, 1996). This investigation found no shade preference during routine nudibranch behaviours, though it would be of interest to determine if egg ribbon depository sites were selected with shade preference in the laboratory and in nature.
CONCLUSION:
Only egg ribbons of A. montereyensis of the five nudibranchs studied in this paper have been observed by others to be preferentially deposited in shaded conditions over lighted ones in intertidal ecosystems. This observation has yet to have been made for the other species or in ecosystems at depths of 6m and greater. This preferential depositing in and group preference for shade is obviously characteristic of the San Juan Island intertidal populations of A. montereyensis, thus the generalizations made by Geiger and Holyoak (1996) during their work there are too broad when dealing with such a specific population. Again, it must be clear that shade preference may not be as one-sided as a preference over lighted conditions. Underlying factors of protection from current and desiccation amongst others may play a role. It should be said instead that dorid nudibranchs in littoral (intertidal) ecosystems have adapted to express a preference for shaded conditions in general and for the deposition of their egg ribbons in specific, while populations of dorids in deeper water ecosystems that are not selected against in terms light or dark condition predilection, do not show such a preference.
ETHICAL CONSIDERATIONS:
All specimens used for this experiment were obtained at the Race Rocks Marine Protected Area, southwest of Victoria, BC. Being the first ecological reserve of its kind in Canada, all organisms contained within its boundaries are protected; therefore it was of paramount importance that no harm was done to these nudibranchs, and that they were returned as close to where they were obtained as possible. Any species obtained from the Race Rocks MPA was used in conjunction with Lester B. Pearson College’s outreach education programme, to educate school-aged children about the marine life around them. Individuals of all species used in this programme are treated in a similar fashion, not being away from their natural habitat for more than three weeks.
None of the nudibranch species studied are keystone species[9]. They have no known predators, and most of the species selected have a highly specialized diet of H. panicea. For these reasons, though it is important for these organisms to be in their environment, it was not a significant ecological disruption for the duration of the experiment.
REFERENCES:
Beeman, R.D. and Williams, G.C. (1980). Opisthobranchia and Pulmonata: the sea slugs and allies. Pp. 308-354 in R. H. Morris, D. P. Abbott & E.C. Haderlie (eds), Intertidal Invertebrates of California. Stanford University Press: Stanford, California.
Behrens, D.W. (1991) Pacific Coast Nudibranchs. Sea Challengers: Monterey,
California.
Bertsch, H. (1999). Nudibranchs: Marine Slugs With Verve. http://siolibrary.ucsd.edu/slugsite/nudi_han.htm
Biermann, C.H., Schinner, G.O., and Strathmann, R.R. (1992). Influence of solar radiation, microalgal fouling, and current on deposition site and survival of embryos of a dorid nudibranch gastropod. Marine Ecology Progress Series 86(3):205-215.
Damkaer, D.M., Dey, D.B., Heron, G.A., and Prentice, E.F. (1981). Effects of UV-B radiation on near surface zoo plankton of Puget Sound. Oecologia 48: 178-182.
Elvin, D.W. (1976). Feeding of a dorid nudibranch, Dialulula sandiegensis, on the sponge Haliclona permollis. Veliger 19:194-196.
Geiger, H.L. and Holyoak, A.R. (1996). Preference of Adults of the Dorid Nudibranchs Archidoris montereyensis (Cooper, 1862), Diaulula sandiegensis (Cooper, 1862), and Triopha catalinae (Cooper, 1863) for Shaded Over Lighted Conditions. Veliger 39(1):95-97.
Goddard, J.H.R. (1984). The opisthobranchs of Cape Arago, Oregon, with notes on their biology and a summary of benthic opisthobranchs known from Oregon. The Veliger, 27(2): 143-163.
Goddard, J.H.R. (1998). A summary of the prey of nudibranch molluscs from Cape Arago, Oregon. Opisthobranch Newsletter, 24: 11-14.
Hadfield, M.G. (1986). Settlement and recruitment of marine invertebrates: a perspective and some proposals. Bulletin Marine Science 39: 418-425.
Hurst, A. (1967). The egg masses and veligers of 30 Northeast Pacific opisthobranchs. Veliger 9: 255-288.
Katz, P. (2000). Research Emphasis: Neuromodulation of Central Pattern Generator
Circuits. http://www.mcg.edu/SOM/mdphd/katz.htm
Kirkmann, T. Paired Student’s t-Test. http://www.physics.csbsju.edu/stats/Paired_t-test_NROW_form.html
McDonald, G.W. (1983). A review of the nudibranchs of the California coast. Malacologia, 24: 114-276.
McDonald, G.R. and Nybakken, J.W. (1978) Additional notes on the food of some California nudibranchs with a summary of known food habits of California species. Veliger, 21(1): 110-119.
Morris, R.H., Abbott, D.P., and Haderlie, E.C. (1983). Intertidal Invertebrates of California. Stanford University Press, Stanford, California: 323-331.
Morrow, C.C. and Picton, B.E. (2000). Nudibranchs of the British Isles: Anatomy. http://www.pictonb.freeserve.co.uk/nudibranchs/anatomy.html
Pechenik, J.A. (1986). The encapsulation of eggs and embryos by molluscs: an overview. American Malacological Bulletin 4: 165-172.
Pidwirny, M.J., (2000). Fundamentals of Physical Geography. http://www.geog.ouc.bc.ca/physgeog/physgeoglos/i.html
Poizat, (1985). Interstitial opisthobranch gastropods as indicator organisms in sublittoral
sandy habitats. Malacology. 35: 17.
Rosenfeld, J. (2000). The Vibrant Sea http://www.oz.net/~vibrant/nudibranchs.html
Rumrill, S.S. (1990). Natural mortality of marine invertebrate larvae. Ophelia 32: 163-198.
Smallwood, W. M. and C. G. Rogers. (1908). Studies on nerve cells.
1. The molluscan nerve cell, together with summaries of recent literature on the cytology of invertebrate nerve cells. Journal of Comparative Neurology & Psychology 18:45-85.
Thorson, G. (1964). Light as an ecological factor in the dispersal and settlement of larvae
of marine bottom invertebrates. Ophelia 1: 167-208.
Vogel, S. and LaBarbera, M. (1978). Simple flow tanks for research and teaching. Bioscience 28: 638-643.
Werry, K.L. (1996). Ringed Dorid. http://www.clever.net/kerry/creature/ringed.htm
[1] Cerata are long finger-like projection originating from the mantle of aeolid nudibranchs. They often function as forms of chemical defense, and are utilized in respiration not unlike the branchial plume in dorids.
[2] the space between individuals ranged from 15 to 25 cm apart, depending on the number of specimens in the run.
[3] These occurred on November 7, 2000.
[4] One specimen spent 10 minutes in the dark end of the tank, and then the remaining 30 minutes in the light, see Figure 5.
[5] Test runs, lasting 5 minutes, were conducted immediately after a nudibranch had been disturbed (removed from the bottom). Initial condition preference was noted for the experiment. It was observed though, that overnight, most (71.4%) individuals had migrated towards the seawater flow (into the current), even if they had shown a preference against it during experimentation.
[6] The simple flow tank used for experimentation had a drain at one end.
[7] leaf-like part of the algae
[8] marine bottom-dwelling organisms
[9] Keystone species are species that don’t interact with a large number of other species in their community, interactions so important that the removal of this species could cause widespread changes to community structure.